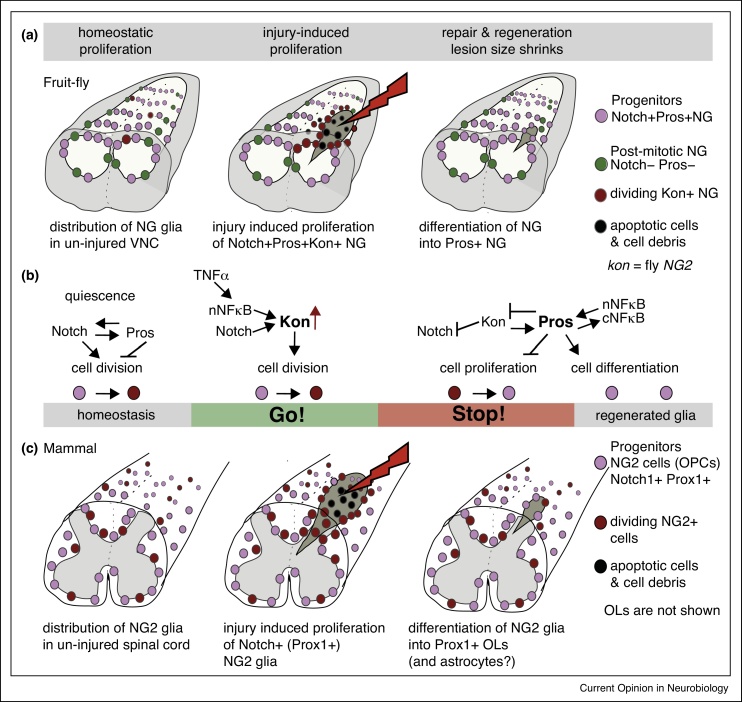
Figure 1.
The glial regenerative response to CNS injury in fruit-flies and mammals. (a) The Drosophila larval ventral nerve cord. The Notch+Pros+NG (mauve) have cell bodies surrounding the neuropile, with part of their cytoplasms extending into regions of the neuropile, where axons and dendrites are located (white). Neuronal cell bodies and other glial cell types are located in the cortex (grey). Notch+Pros+NG divide during axon guidance, but they rarely divide in larval life, which lasts for five days. Divisions in larva are homeostatic. Upon injury to the larval VNC, the lesion first expands as many cells die. Injury induces compensatory proliferation of surviving NG. Subsequently, proliferation ceases, glial cells differentiate and the lesion shrinks. (b) Molecular mechanism underlying the glial regenerative response in Drosophila. NG are kept ‘ready to divide’ through the mutually dependent, yet antagonistic functions of Notch and Pros. Injury induces the activation of NFκB/Dorsal and Notch-dependent up-regulation of kon (homologue of NG2) expression. Kon induces proliferation of Notch+Pros+NG. Kon also activates the expression of pros. Pros inhibits proliferation and activates glial differentiation. Negative feedback by Kon on Notch, and by Pros on kon expression, terminates the response to injury. Pros regulates the expression of NFκB/dorsal, which remains in the cytoplasm ready to respond to future injuries. (c) The mammalian dorsal spinal cord. NG2 glia populate the white matter, that is, neuropile with myelinated axons (in white). Some of these NG2 glia normally divide producing meylinating oligodendrocytes (OLs, not shown). Injury induces cell death, lesion expansion and subsequent compensatory proliferation of remaining NG2 glia. Newly produced glia can then differentiate into astrocytes or OLs, and can spontaneously re-myelinate axons, as the lesion shrinks. Genes and resulting molecular mechanisms in these responses are evolutionarily conserved.