Table 1.
Heterocyclic derivatives in RAR α, β and γ transactivation assays.a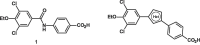
| Compd | Het | ∝ EC50 nMa | β EC50 nMa | Fold selectivity for β over αb | γ EC50 nMa | Fold selectivity for β over γb | cLogPd |
|---|---|---|---|---|---|---|---|
| ATRA - | 1.9 | 1.2 | 1.56 | 0.9 | 0.75 | ||
| 1 | – | 46 | 1227 | 0.037 | 30,000 | 24 | 4.4 |
| 2 | 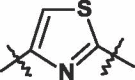 |
240c | 120 | 2 | 160 | 1.3 | 6.1 |
| 3 | 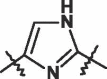 |
594c | 423 | 1.4 | ND | – | 5.6 |
| 4 | 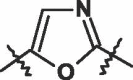 |
60 | 28 | 2.1 | 45 | 1.6 | 5.5 |
| 5 | 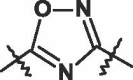 |
18c | 1.5 | 12 | 28 | 19 | 5.1 |
| 6 | 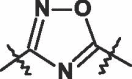 |
31 | 110 | 0.28 | 5.4 | 0.05 | 5.1 |
| 7 | 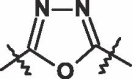 |
58 | 63 | 0.92 | 150 | 2.4 | 4.3 |
Transactivation assays for the RAR alpha, beta and gamma receptors were performed using each of the mouse RAR ligand binding domains. Values usually obtained from three separate experiments. Errors in these assays are approximately 20% of the mean values. Transactivation Assays details see Supplementary data and reference 4. ATRA is all trans retinoic acid.
The EC50 ratios of ∝ to β and γ to β.
Compound behaves as a partial agonist relative to the amplitude of the normalising ATRA output. All other compounds were determined to be full agonists with their maximum upper asymptote within 20% of that found for ATRA.
Ref. 9.
