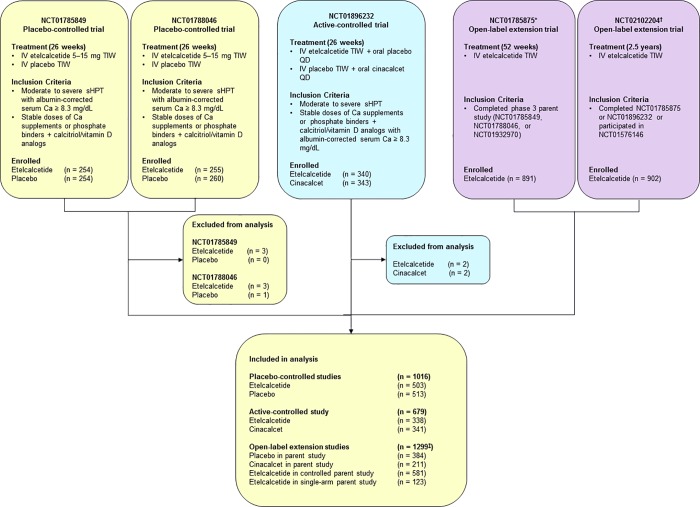
Fig 1. Trials included in the integrated safety analysis.
*Trial NCT01785875 also contains data from patients enrolled from a single-arm parent trial (i.e., trial NCT01932970). †Trial NCT02102204 also contains data from patients enrolled from a phase 2 parent trial (i.e., trial NCT01576146). ‡Indicates unique patients in the OLE pool. Ca = calcium; QD = every day; IV = intravenous; sHPT = secondary hyperparathyroidism; TIW = three times weekly.