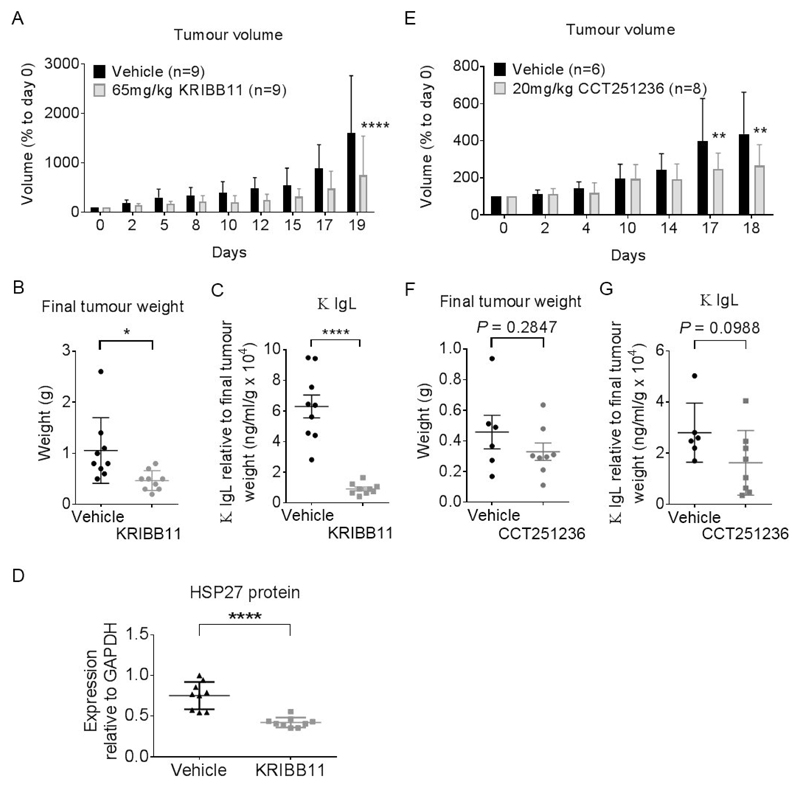
Figure 6. KRIBB11 and CCT251236 are efficacious in a subcutaneous H929 human myeloma xenograft model.
Athymic mice bearing well-established tumors were dosed daily with 65 mg/kg KRIBB11 i.p. or 20 mg/kg CCT251236 p.o. or vehicle over 19 and 18 days, respectively. At the end of the study, 16 hours after the final dose, plasma was collected and tumors were harvested and weighed. (A) Mean tumor volume as a percentage of day 0 (start of treatment). Statistical differences were calculated by two-way ANOVA followed by Bonferroni’s multiple comparisons test with FWER ≤ 0.05. (B) Mean final tumor weights. (C) Mean κ Ig light chains detected in plasma samples. Values are normalised to final tumor weight. (D) Mean HSP27 protein expression as determined by densitometry analysis of Western blot bands relative to GAPDH loading control presented in Supplementary Figure S7B. Data are shown as means ±S.D. Statistical differences were calculated by Student’s t-tests. P-values are indicated where * ≤ 0.05, ** ≤ 0.01 and **** ≤ 0.0001.