Table 1. IC50 and Ki Values and Selectivity Data for 5 and 10a–f.
| IC50(nM)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| R-NH2 | Inhibitor Type |
Ki (nM) MMP-13 |
MMP-13 | MMP-1 | MMP-2 | MMP-8 | MMP-9 | MMP-14 | |
| 5 | – | 800 | 2400 | -b | -b | -b | -b | -b | |
| (S)-10a | 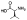 |
Zn2+ chelator | 2.3 | 2.2±0.8 | -c | -c | -c | -c | -c |
| (R)-10a | 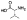 |
Zn2+ chelator | 1.6 | 7.0±1.2 | -c | -c | -c | -c | -c |
| 10b | 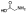 |
Zn2+ chelator | 1.8 | 1.6 | -c | -c | -c | -c | -c |
| 10c | 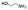 |
non-chelator | 9.2±2.0 | 12.0±2.2 | 4000 | >5000 | >5000 | >10000 | >10000 |
| 10d | 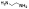 |
non-chelator | 13.5±37 | 3.4±0.4 | >5000 | 730 | 600 | >10000 | >10000 |
| 10e | 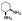 |
non-chelator | - | 17.9±1.2 | >10000 | 2700 | 3500 | >10000 | >10000 |
| 10f | 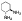 |
non-chelator | - | 16.9±1.0 | >10000 | >10000 | >10000 | >10000 | >10000 |
The IC50 values were determined by using fluorescence resonance energy transfer triple-helical peptides (fTHP) as substrates in the enzyme assay.24,30,31
Compound 5 was tested against MMP-1, -2, -8, -9, and -14 at a single concentration and these data are reported in ref 28 (Roth et al.).
Because (S)-10a, (R)-10a, and 10b are expected to be Zn-chelating agents, these were only tested at a single concentration; these data are reported in Supporting Information, Figure S1. Determination of inhibition constants and modalities were conducted by incubating the range of fTHP-15 substrate concentrations (2–25 μM) with 4 nM MMP-13 at room temperature in the presence of varying concentrations of inhibitors (0.5–50 nM). Experiments were performed 1–3 times in duplicates or triplicates.
