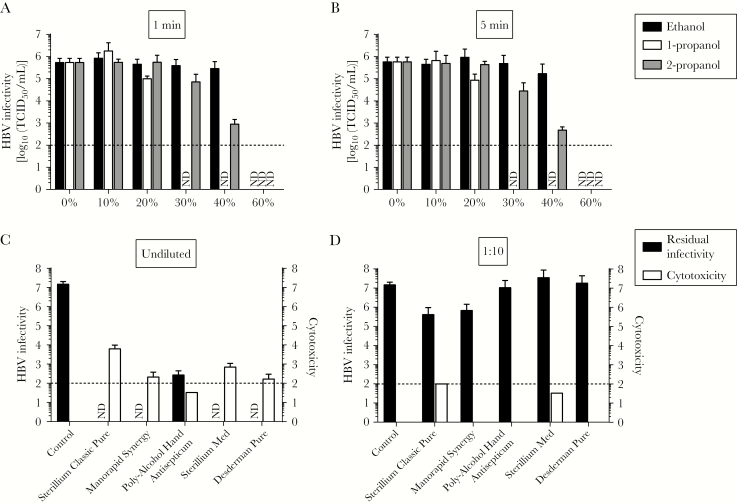
Figure 2.
Inactivation of hepatitis B virus (HBV) by different kinds of alcohol and commercial hand antiseptics. A and B, Ethanol, 1-propanol, and 2-propanol were tested for their efficacy in inactivating HBV. The biocide concentrations ranged from 0% to 60%, with exposure times of 1 minute (A) or 5 minutes (B). For this inactivation assay, 1 part virus and 1 part bovine serum albumin were mixed with 8 parts biocide. Residual infectivity was determined by a limiting dilution assay. Viral titers are displayed as half-maximal tissue culture infective doses (TCID50). Cytotoxicity was determined by examining permissive cells by microscopy for any significant changes in the cell monolayer and was calculated analogously to virus titers (data are TCID50/mL). Data are mean values (±SDs) of 3 independent experiments. ND, no residual infectivity detected. C and D, Five commercial hand antiseptics (Sterillium Classic Pure, Manorapid Synergy, Poly-Alcohol Hand Antiseptic, Sterillium Med, and Desderman Pure) were tested in a quantitative suspension assay for their efficacy in inactivating HBV, as described above. Exposure times of 30 seconds were used at concentrations of 80% (C) and 8% (D). Residual infectivity was determined by a limiting dilution assay (ie, the TCID50 assay). Cytotoxicity was determined by examining permissive cells by microscopy for any significant changes in the cell monolayer and was calculated analogously to virus titers (data are TCID50/mL). Data are mean values (±SDs) of 3 independent experiments. ND, no residual infectivity detected.