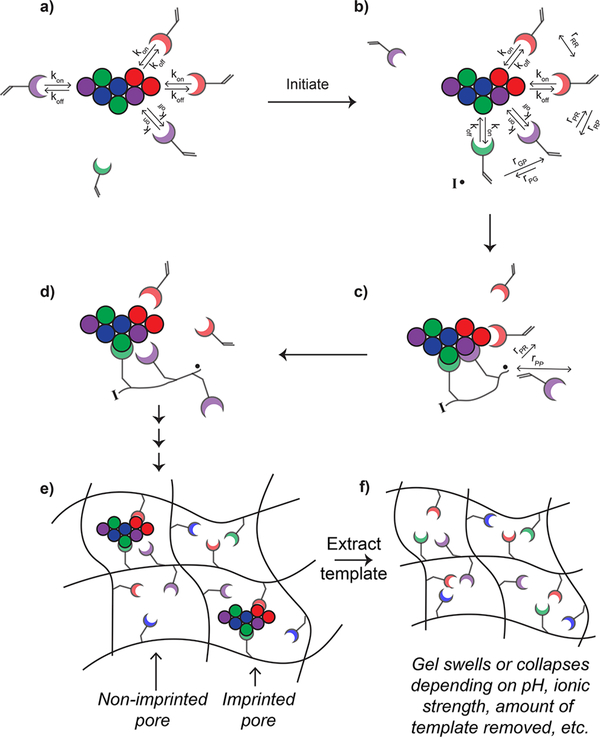
Figure 3.
Schematic depiction of the dynamic nature of noncovalent protein imprinting. (a) Interactions between monomer and template protein are highly dynamic. The residence time (i.e., time that the monomer is bound to the protein) depends on the off rate (koff) and is dependent on the strength of the noncovalent interactions between the monomer and protein. The on rate (kon) is a function of diffusion as well as long-range electrostatic interactions. (b) Upon initiation, the “pre-assembled” system may look different than just moments before initiation. The ability of monomers to polymerize together is determined by both localization and reactivity ratios of the monomers. Long and short arrows depict high and low preference, respectively, for reacting with the nearby monomer. (c) If two monomers are in proximity to the growing radical oligomer, the monomer with which the radical reacts will be influenced by the reactivity ratios. (d) By the time the next propagation step is occurring, the monomers or growing oligomers may diffuse away from the protein template, although higher molecular weight species will have a slower koff (i.e., a longer residence time) due to the multivalent nature of the interaction. (e) After the polymerization is complete, some of the pores in the hydrogel are imprinted while others are not, leading to nonspecific binding sites. (f) After the template is extracted, the MIP is likely to swell or collapse depending on the solvent, environmental conditions (e.g., pH, ionic strength), and amount of template removed.