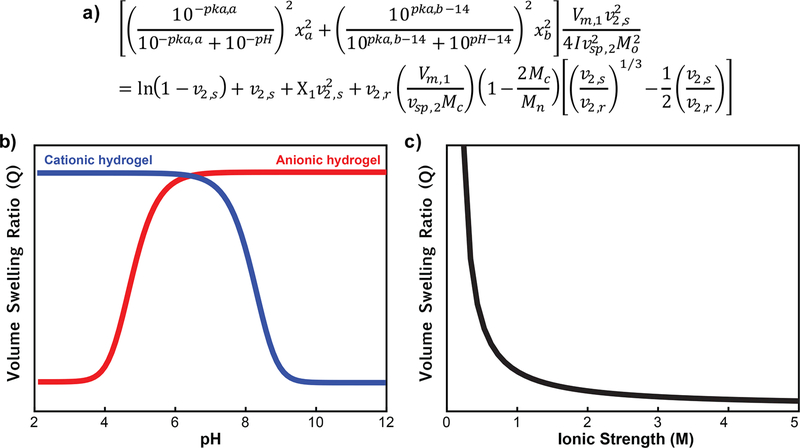
Figure 4.
Swelling behavior of ionizable hydrogels. Equilibrium volume swelling ratio (Q) calculated as a function of pH or ionic strength using the modified Brannon—Peppas equation. (a) Q for anionic hydrogels increases as the hydrogels transition above the pKa of the acidic group, while Q for cationic hydrogels increases as they transition below the pKa of the basic group. (b) Q decreases rapidly with increasing ionic strength. This swelling behavior is important to keep in mind during template extraction and rebinding steps.