Figure 1. Biophysical principles and the results of the HTS for CRBP1 ligands.
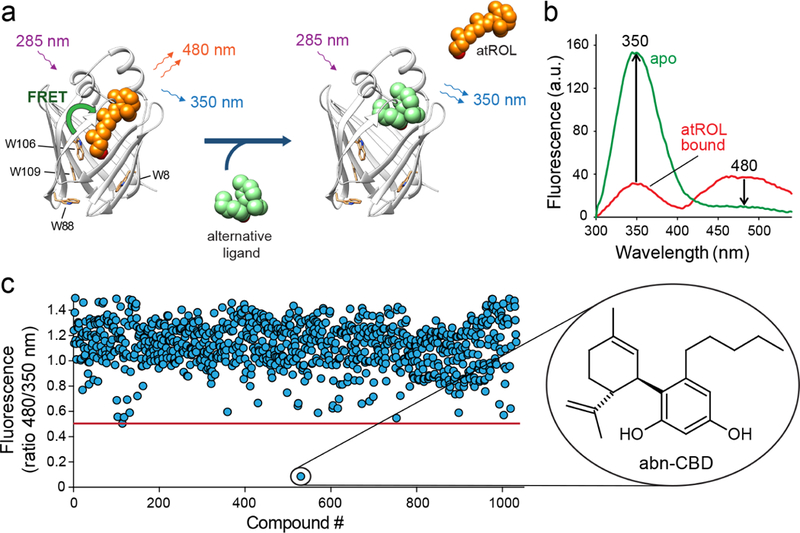
(a) A schematic representation of the vitamin A-displacement assay. Replacement or liberation of atROL from holo-CRBP1 by an alternative nonretinoid ligand results in diminishing of FRET between the retinoid moiety and the protein scaffold. (b) Differences in the fluorescence emission spectra between CRBP1 in complex with atROL and the apo form of the protein were used as a readout in a high-throughput assay. (c) The primary screening of a chemical library composed of bioactive lipids revealed a single hit that corresponded to a synthetic derivative of cannabidiol, abn-CBD.
