Abstract
Background and objectives
Infants who begin life in the medicalized environment of the neonatal intensive care unit (NICU) do so under stressful conditions. Environmental exposures are often abrasive to vulnerable infants, while invasive and noninvasive lifesaving interventions provide additional pain and/or stress. The most commonly selected biomarker to measure stress is cortisol. The skin is the barrier between the external environment and communicates with our neurological, endocrine and immune regulatory networks. To examine if skin cortisol may be a reliable biomarker of stress, NICU stress exposure and repeated measurements of skin cortisol in very preterm infants were examined prospectively during the first 6 weeks of life. The temporal relationship between skin cortisol and NICU stress exposure was also analyzed.
Materials and methods
Participants included 82 preterm infants born weighing less than 1500 grams, admitted to a level III NICU, with a mean gestational age of 28.5 weeks. Infants were studied from birth through 6 weeks of life. NICU stress data was collected using the Neonatal Infant Stressor Scale. Skin samples were collected using D-squame tape as soon after birth as possible and every two weeks thereafter.
Results
On average, infants experienced approximately 43 stressful events per day during the first 6 weeks of life in the NICU. Stress level and cortisol reactivity varied by gestation age. Higher stress resulted in higher cortisol for infant >28 weeks; lower stress scores were associated with higher stress for infants <28 weeks. Stress exposure during 7 days prior to cortisol sampling yielded the highest AUC for the 2 groups. A statistically significant interaction was identified between gestational age and stress exposure during the previous 7 days (p < 0.01).
Conclusion
This is the first study to demonstrate skin cortisol as a preterm infant biomarker of chronic stress exposure. For infants with appropriate skin maturation, this non-invasive sampling method provides several benefits. Importantly, this method may be less intrusive and disruptive for preterm infants.
Keywords: NICU, Preterm infants, Cortisol, Skin cortisol, Stress
1. Introduction
Infants who begin life in the medicalized environment of the neonatal intensive care unit (NICU) do so under conditions very different from that of healthy term-born infants. Environmental exposures (i.e. light, sound, touch) are often abrasive and stressful to vulnerable infants, while various invasive and noninvasive lifesaving interventions are often painful and/or stressful (Cong et al., 2017; Lai and Bearer, 2008; Newnham et al., 2009). Depending on the severity of illness and/or immaturity at birth, many of these interventions are repeated over and over during the course of care. Additionally, during the NICU experience, parents are supplanted by clinicians as the primary caregivers. Within the context of one’s medical condition, this collective experience of pain, stress and parent-infant separation has been described as Infant Medical Trauma in the NICU (IMTN; (D'Agata et al., 2016)). These treatment-related experiences fit the classic definition of uncontrolled stress exposure or toxic stress (Shonkoff et al., 2011). Accumulating evidence demonstrates linkages between early life experience and long-term health outcomes (Duerden et al., 2018; Sullivan et al., 2012; Sullivan et al., 2018).
Preterm infants who endure chronic stress during this highly vulnerable period of development are at increased risk for abnormal brain development (Fumagalli et al., 2018; Joels and Baram, 2009; Smith et al., 2011). Vinall et al. (2014) found school age children born as very preterm infants experienced more than 74 invasive procedures during NICU care and had altered white matter microstructure (Vinall and Grunau, 2014). Recent research suggests preterm infants may also be at increased neurodevelopmental risk from early life NICU stress and pain exposure because of biological susceptibility (Chau et al., 2015; D'Agata et al., 2017).
The most commonly selected biomarker to measure stress response, both acute and chronic, is cortisol. Cortisol, a steroid hormone produced in the adrenal cortex and regulated by the hypothalamic-pituitary-adrenal axis (HPA), that exerts systemic and adaptive effects. Some of the effects include blood pressure regulation, inflammation and immune response reduction and water retention. Acute cortisol levels and its precursors have been investigated in critically ill term and preterm infants. In term infants with and without therapy-resistant hypotension from septic shock, Khashana et al. (2016) examined the physiological response of serum cortisol and several cortisol precursors. While investigators found no differences in cortisol between the two groups, newborns with treatment resistant hypotension had significantly higher amounts of the precursor 3-beta-hyrdoxysteroid dehydrogenase, whereby limiting cortisol synthesis (Khashana et al., 2016). Limited cortisol synthesis has also be found in the serum of extremely preterm infants who were ill during the first week of life (Hingre et al., 1994).
The studies mentioned above and others have used serum cortisol sampling to provide evidence of acute stress exposure. Use of serum dependent biomarkers in preterm infant research is challenging because of concerns about unnecessary depletion of blood volume and the infliction of additional pain. To avoid these factors, serum alternative biomarkers that reliably measure stress are desired. Because cortisol is the biomarker most commonly selected in research studies to measure stress, noninvasive sources are of interest. To date the serum alterative biological sources that measure acute stress include saliva and urine (Moore, 2015; Vittner et al., 2017). Biological sources of cortisol that measure chronic stress include hair and nail sampling (Bates et al., 2017; Binz et al., 2018; Groer et al., 2014). Pragmatic factors that complicate collection of these samples in preterm infants include very limited hair (Stalder et al., 2017) and nail growth and saliva sample collection itself may be noxious due to limited volume (Maas et al., 2014; Mitchell et al., 2012). Due the inherent sample collection challenges of these noninvasive methods, alternative options are of continued interest.
Skin is the body’s physical barrier between the external environment, with its multiple dangers and threats, and the internal environment. The skin communicates with our neurological, endocrine and immune regulatory networks. Additionally, the skin is richly innervated with sensory nerves and has an extensive blood supply so there is constant communication between multiple regulatory systems and the skin (Zmijewski and Slominski, 2011). The skin “HPA” may in fact coordinate the initial response to environmental stressors (Narendran et al., 2010).
Maintenance and protection of skin integrity for these infants is an essential nursing function. Premature infant skin is thinner than term infant skin, characterized by an immature stratum corneum (Evans, 1986; Rutter, 1996). This puts the preterm infant at risk for infection, dehydration, electrolyte imbalances and poor thermoregulation. Investigators have characterized the skin of premature infants, gestational age less than or equal to 32 weeks, as “wounded” (Narendran et al., 2010). The process of preterm infant skin maturation requires skin to mature over several weeks after birth in order to achieve the more complete barrier provided by term infants’ skin (Nonato et al., 2000). All skin contains an active innate immune system and hair follicles that respond to corticotropin-releasing hormone (CRH). This occurs by upregulation of the proopiomelanocortin (POMC) gene transcription, production of both, adrenocorticotropic hormone (ACTH) and α-melanocyte stimulating hormone. The follicle responds, in in-vitro culture, by releasing cortisol (Ito et al., 2005). This local CRH-responsive cortisol release is a peripheral equivalent of the HPA, demonstrating that not only cells in the hair follicle can produce cortisol. Melanocytes and other skin cells have also been shown to have this capability (Zmijewski and Slominski, 2011).
The only known investigation of cortisol from preterm infant skin surface sampling is by Narendran et al. (2010), who found skin to be a biomarker of innate immunity from a single sample during the first week of life (Narendran et al., 2010). Investigators suggested elevated skin cortisol may have been a neuroendocrine response to the stress of preterm birth.
We hypothesized that preterm infants with higher NICU stress exposure would have higher skin cortisol concentrations. Due to skin development and sloughing processes, we anticipated that higher cortisol concentrations would be associated with higher NICU stress events occurring less than one month from sampling, yet the exact timing was unclear. In this exploratory study, we first aimed to examine if skin cortisol may be a reliable biomarker of chronic stress and secondly, we investigated the temporal relationship between skin cortisol and NICU stress exposure. We designed our study to retrospectively investigate the relationship between NICU stress exposure and repeated measurements of skin cortisol in very preterm infants during the first 6 weeks of life.
2. Materials and Methods
2.1. Study sample
Participants included 82 preterm infants born weighing less than 1500 grams, admitted to the level III NICU in 2011-2013 at Tampa General Hospital in Tampa, Florida. Exclusion criteria for study entry included birth weight greater than 1500 grams, major congenital anomalies, moribund infants and infants of HIV-infected mothers. The study was approved by the University of South Florida Institutional Review Board. Infants were enrolled after informed consent was obtained from their parent or guardian.
2.2. Procedures
The infants were recruited by a NICU-trained research nurse. Infants were studied from birth through 6 weeks of age, with data from the hospital course collected daily from the electronic health record. Stress data were collected from birth through day 42 of life, or until discharge from the NICU, whichever came first. Once skin was determined to be sufficiently mature for sample collection, samples were collected approximately every two weeks for 6 weeks.
2.3. Measurements
2.3.1. Stress
Stress exposure was measured using a modified Neonatal Infant Stressor Scale (NISS) (Newnham et al., 2009), as has been used on other studies (Cong, 2016; D'Agata et al., 2017; Xu et al., 2016). The NISS is a ranked quantitative instrument used to collect information concerning daily stress experiences of NICU infants during care. Interventions performed on infants are measured, not infant response. Daily stressors are tallied for a daily stress score. Data is typically collected from the infant’s medical record. Common NICU interventions are ranked on a 4-point scale as extremely stressful, very stressful, moderately stressful and a little stressful. Each procedure attempt is counted as one stress event. Examples of NICU care procedures and their corresponding scoring include: each intubation attempt is extremely stressful and weighted with a score of 5; each suctioning attempt is very stressful, score of 4; diaper change is moderately stressful, score of 3; and aspiration of nasogastric tube is a little stressful, score of 2.
Infants receiving care in the NICU typically encounter a common core of interventions performed daily, regardless of health status. Examples of these interventions may include daily weight, diaper changes, position changes and gavage feedings. Many of these interventions are repeatedly performed each day at regular intervals. Greater variability often occurs with higher intensity stressful and painful interventions, such as intubation, blood draws and suctioning. As a result, we analyzed approximately 850 days of previously collected NISS data (D'Agata et al., 2017) from another NICU to develop a core score of basic stressors. By averaging 850 days of a little stressful and moderately stressful NISS scores (D'Agata et al., 2017), we identified 109 as a core score for very preterm infants. Next, we randomly selected 15 participants from this study and collected the same a little stressful and moderately stressful data over 42 days (approximately 630 days of data) to compare findings. Despite the NICU setting for these two cohorts being 2 different states in different regions of the United States, a core score of 109 was identified. This 109-core score for each cohort is a weighted NISS score that reflects 40 daily stress events. Added to the core score from the medical record were the actual attempted and completed higher intensity stress events, very stressful and extremely stressful, for a daily stress score. Analyses include both weighted NISS stress scores and unweighted event counts.
2.3.2. Cortisol
For infants born less than 28 weeks gestational age, skin integrity and maturity was assessed by a neonatologist to determine if skin testing could be safely performed, as described by Narendran et al. (Narendran et al., 2010). If the skin was very immature and not keratinized, as may occur in an extremely low birth weight infant, the tape was not applied. Because the skin of the preterm infant matures rapidly after birth, the integrity was reassessed every 48 hours. Skin sampling was performed as soon after birth as possible, proceeding every two weeks thereafter. Samples were planned for collection every 2 weeks for 6 weeks, ideally resulting in 3 samples/infant. For infants whose skin were not suitably mature for dermatologic tape application, sampling was delayed 1-2 weeks. For some infants this delay resulted in sampling into week 7 of life (n=4).
Early in the study, assay optimization and technical issues with equipment occurred which consumed greater than anticipated specimen volumes. While some samples were unable to be collected due to the medical condition of infants during scheduled sampling or other unforeseen issues, these were minor issues. The majority of missing data occurred for laboratory reasons.
Samples of skin were collected through the use of 380 mm2 D-squame tape (CuDerm, Dallas, Tx) applied to the chest or abdomen with consistent pressure, removed after 2 minutes and stored at −80° C. This sampling method obtained the outermost layer of the stratum corneum. The tape was thawed and added to PBS, 0.2%SDS, and 0.5% propylene glycol and sonicated for 60 minutes at 4°C. Total soluble protein was determined with a modified Lowry assay and cortisol levels were normalized to protein and are reported as pg/ml. The Milliplex Human Skin Magnetic Bead Panel Multiplex Kit (Millipore Sigma, Darmstadt, Germany) was used to measure cortisol. All multiplex kits were prepared according to manufacturer’s directions and read on a Magpix.
2.4. Statistical analysis
Descriptive statistics were generated in the form of means with standard deviations (sd) for continuous variables and counts with percentages (%) for categorical variables to characterize the sample. The distribution of cortisol measurements was examined for normality. A square root transformation was used to normalize the positively skewed cortisol distribution. We hypothesized that skin cortisol concentrations are a function of the historical stress exposure context each infant. Little guidance was able to be found in the literature regarding the duration of time that might be associated with skin sampling. Given this, we examined stress exposure that occurred during 4 time periods prior to the cortisol measurement: 3 days prior to sampling, 7 days prior to sampling, 14 days prior to sampling and all days prior to sampling. To assess how well each of these stress exposure durations were associated with higher cortisol concentrations, receiver operating curves (ROC) were used. The optimal duration was selected based on the largest area under the curve (AUC) estimates derived from the ROC analysis.
We examined the association of cortisol with potential maternal/neonatal covariates. A similar analysis was conducted with average prior stress scores and potential covariates. Using the selected stress duration from the ROC analysis, the association of skin cortisol and average prior stress was examined adjusting for day of sample and other potential covariates (infant gender, race, maternal smoking, delivery complications, gestational age) using generalized estimating equations (GEE). The GEE method allows us to examine the association of skin cortisol and average prior stress adjusting not only for the covariates but also accounting for the repeated nature of the data within each infant. To reduce the potential for over parameterization of the model, covariates were only kept in the model if their p-value fell below 0.10.
All analyses were conducted with IBM SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). A two-tailed p-value less than or equal to 0.05 was considered statistically significant.
3. Results
3.1. Sample characteristics
The study population consisted of 82 very low birthweight infants, with a mean GA of 28.5 weeks (range 24 to 37 weeks), 53% male (n = 40). Four infants dropped out of the study and 3 infants did not have samples collected, resulting in 75 infants with available samples. Twenty-three of the 75 were multiples (10 sets of twins and 1 set of triplets). All but two of these infants were born at the study hospital. Seventy-seven percent were delivered by cesarean section. Average length of stay was 68 days (range 20 – 215 days). Maternal and infant characteristics are presented in Table 1.
Table 1.
Sample Characteristics by GA Group.
| Birth Gestational Age | ||||
|---|---|---|---|---|
| Characteristic | Overall n (%) |
GA≥28 weeks n (%) |
GA<28 weeks n (%) |
p |
| Infant gender | 0.02 | |||
| male | 40 (53.3) | 18 (41.9) | 22 (68.8) | |
| female | 35 (46.7) | 25 (58.1) | 10 (31.2) | |
| Maternal prenatal preeclampsia | 22 (29.3) | 16 (37.2) | 6 (18.8) | 0.08 |
| Maternal prenatal abruption | 7 (9.3) | 2 (4.7) | 5 (15.6) | <0.01 |
| Maternal prenatal PTL, PROM, PPRM | 46 (61.3) | 18 (41.9) | 28 (87.5) | 0.13 |
| Maternal prenatal chorio | 4 (5.3) | 1 (2.3) | 3 (9.4) | 0.31 |
| Maternal prenatal smoking | 10 (13.3) | 7 (16.3) | 3 (9.4) | 0.50 |
| Mother's Race/Ethnicity | 0.58 | |||
| Caucasian | 24 (32.0) | 15 (35.7) | 9 (28.1) | |
| African American | 31 (41.3) | 17 (40.5) | 14 (43.8) | |
| Hispanic white | 14 (18.7) | 6 (14.3) | 8 (25.0) | |
| Hispanic black | 1 (1.3) | 1 (2.4) | 0 (0.0) | |
| Asian/PI | 2 (2.7) | 2 (4.8) | 0 (0.0) | |
| Other | 2 (2.7) | 1 (2.4) | 1 (3.1) | |
| Infant Race/Ethnicity | 0.30 | |||
| Caucasian | 19 (25.3) | 13 (31.0) | 6 (18.8) | |
| African American | 32 (42.7) | 18 (42.9) | 14 (43.8) | |
| Hispanic white | 15 (20.0) | 7 (16.7) | 8 (25.0) | |
| Hispanic black | 3 (4.0) | 1 (2.4) | 2 (6.3) | |
| Asian/PI | 3 (4.0) | 3 (7.1) | 0 (0.0) | |
| Native American | 1 (1.3) | 0 (0.0) | 1 (3.1) | |
| Other | 1 (1.3) | 0 (0.0) | 1 (3.1) | |
| Birthweight group | <0.01 | |||
| BW 1200+ grams | 25 (33.3) | 23 (53.5) | 2 (6.2) | |
| BW < 1200 grams | 50 (66.7) | 20 (46.5) | 30 (93.8) | |
| GA at birth group | --- | |||
| Birth GA 28+ weeks | 43 (57.3) | 43 (100.0) | 0 (0.0) | |
| Birth GA < 28 weeks | 32 (42.7) | 0 (0.0) | 32 (100.0) | |
| Retinopathy | 0.22 | |||
| None | 65 (86.6) | 39 (90.7) | 26 (81.2) | |
| Stage 1 | 8 (10.7) | 4 (9.3) | 4 (12.5) | |
| Stage 2 | 2 (2.7) | 0 (0.0) | 2 (6.3) | |
| IVH* | 0.41 | |||
| None | 63 (88.7) | 37 (92.5) | 26 (83.9) | |
| Grade 1 | 4 (5.6) | 1 (2.5) | 3 (9.7) | |
| Grades 2-4 | 4 (5.7) | 2 (5.0) | 2 (6.4) | |
| Mean (SD) | Mean (SD) | Mean (SD) | p | |
| Gestational age (weeks) | 28.5 (2.4) | 30.1 (1.9) | 26.4 (0.9) | --- |
| Day of life sample(s) taken | 28.9 (7.9) | 26.8 (8.0) | 31.8 (7.0) | 0.01 |
| Corrected GA (weeks) | 32.7 (2.1) | 33.8 (1.8) | 31.1 (1.4) | |
| Days on O2 | 13.5 (20.8) | 4.1 (8.2) | 26.0 (25.6) | <0.01 |
| Birthweight | 1099 (215) | 1205 (184) | 956 (165) | <0.01 |
| LOS | 68.1 (36.5) | 55.81 (32.2) | 84.7 (35.5) | <0.01 |
4 infants missing IVH documentation (3 GA>28 weeks and 1 GA<28 weeks)
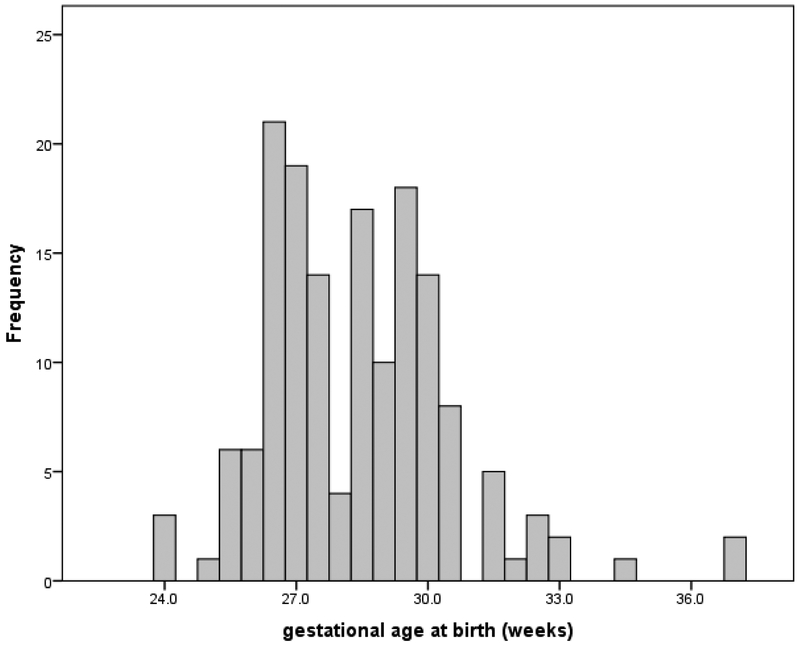
The number of cortisol samples collected by birth gestation age generally dichotomized into 2 groups, greater than or equal to 28 weeks and less than 28 weeks, see Figure 2. In light of this bimodal distribution and published reports of degree of HPA immaturity related to gestational age (Scott and Watterberg, 1995), analyses were conducted using birth age variable of ≥ 28 weeks’ gestation and < 28 weeks’ gestation.
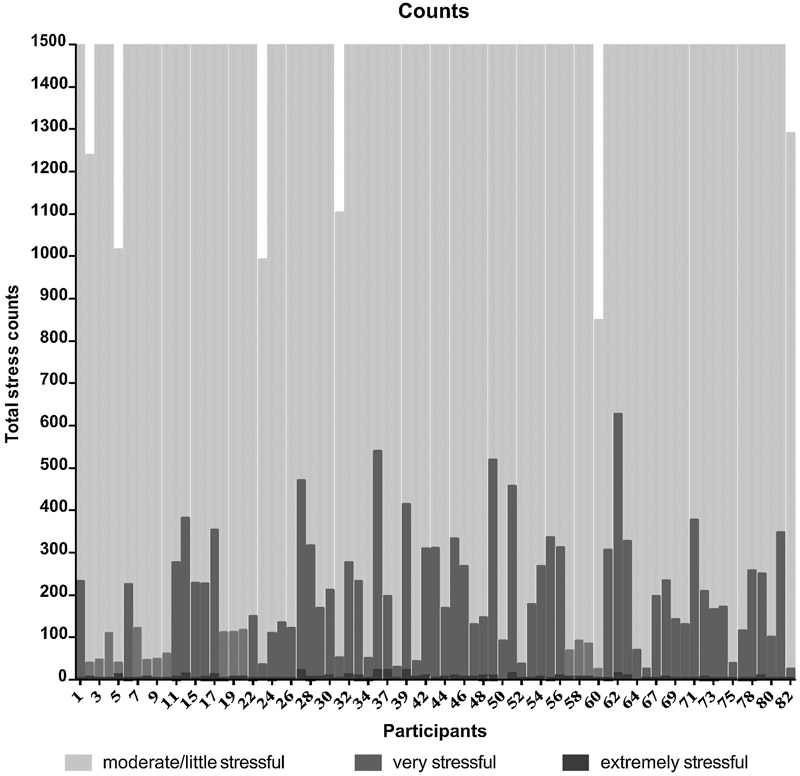
Figure 2.
Counts of Stressor Intensity Over 6 Weeks. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
3.2. Stress
Infants experienced an average count of 1,804 stress events (range 824 – 2,306 events). This indicated approximately 43 stressful events per day. Infants with lower birth gestational age were more likely to have higher counts of stressful events. Mean cumulative stress events by category included: 5 extremely stressful events (range 0 – 22 events); 186 very stressful events (range 17 – 612 events); 1613 moderately and a little stressful events (800 – 1680 events). The mean cumulative NISS score was 5,182 (range 2273 – 7096), suggesting an average daily stress score of 123 (54 – 169). Figure 2 shows the counts of stress by category for each infant. A significant association was found between stress and intraventricular hemorrhage (IVH; p < 0.01, see Table 2).
Table 2.
Association of Skin Cortisol, Average Prior 7 Day Stress Scores, and Sample Characteristics
| Cortisol (pg/ml) | Prior 7 Day Stress Score |
|||||
|---|---|---|---|---|---|---|
| Mean | SE | p | Mean | SE | p | |
| Gender of infant | 0.03 | 0.05 | ||||
| male | 6.206 | 0.389 | 129.00 | 2.03 | ||
| female | 4.766 | 0.508 | 122.68 | 2.48 | ||
| Maternal prenatal preeclampsia | 0.86 | 0.25 | ||||
| No | 5.506 | 0.378 | 127.22 | 1.90 | ||
| Yes | 5.642 | 0.670 | 123.13 | 3.03 | ||
| Maternal prenatal PTL, PROM, PPRM | 0.13 | 0.49 | ||||
| No | 6.124 | 0.451 | 124.81 | 2.26 | ||
| Yes | 5.170 | 0.446 | 127.02 | 2.24 | ||
| Maternal prenatal abruption | 0.84 | 0.02 | ||||
| No | 5.524 | 0.360 | 125.07 | 1.69 | ||
| Yes | 5.683 | 0.683 | 135.77 | 4.40 | ||
| Maternal prenatal chorio | 0.70 | 0.23 | ||||
| No | 5.562 | 0.339 | 126.37 | 1.67 | ||
| Yes | 5.046 | 1.248 | 120.82 | 4.34 | ||
| Maternal prenatal smoking | 0.58 | 0.96 | ||||
| No | 5.473 | 0.356 | 126.18 | 1.72 | ||
| Yes | 5.968 | 0.849 | 125.95 | 4.76 | ||
| GA at birth group | 0.21 | <0.01 | ||||
| ≥ 28 weeks | 5.187 | 0.439 | 119.66 | 1.63 | ||
| < 28 weeks | 5.989 | 0.475 | 134.30 | 2.41 | ||
| Birthweight Group | 0.62 | <0.01 | ||||
| BW ≥ 1200 grams | 5.773 | 0.522 | 118.61 | 1.78 | ||
| BW < 1200 grams | 5.443 | 0.415 | 129.62 | 2.04 | ||
| Infant Race | 0.80 | 0.57 | ||||
| Caucasian | 5.681 | 0.552 | 127.56 | 3.52 | ||
| Black or African American | 5.280 | 0.591 | 124.19 | 2.09 | ||
| Other (mostly Hispanic - 20/28) | 5.786 | 0.493 | 127.44 | 3.04 | ||
| Retinopathy | 0.87 | 0.66 | ||||
| None | 5.524 | 0.362 | 125.83 | 1.70 | ||
| Stage 1-2 | 5.658 | 0.752 | 128.30 | 5.36 | ||
| IVH | 0.38 | <0.01 | ||||
| None | 5.574 | 0.360 | 125.31 | 1.74 | ||
| Grade 1 | 3.920 | 1.153 | 139.90 | 2.27 | ||
| Grades 2-4 | 5.894 | 0.704 | 130.80 | 7.57 | ||
To investigate the epoch of stress exposure represented by the cortisol samples, we examined various durations of time using ROC analysis. The epochs included average stress exposure during the prior 3 days, 7 days, 14 days and cumulative days from birth to sampling day. In the overall sample, AUC values derived from ROC analysis ranged from 0.54 to 0.60 (Table 2). AUC values increased when the sample was analyzed by gestational age (≥ 28 weeks and < 28 weeks). However, the stress level (higher vs. lower) association for higher cortisol levels was different by GA. Of particular note, for infants with GA ≥ 28 weeks, higher stress scores were associated with higher cortisol levels. Whereas, lower stress scores were associated with higher cortisol levels for infants with GA < 28 weeks. Given this pattern dichotomy by GA for higher cortisol levels, the average 7-day prior stress score was deemed a reasonable summary stress measure. The variable for average stress score from the prior 7 days was used in subsequent analyses.
3.3. Cortisol
Skin cortisol samples were collected from 75 preterm infants. Total number of cortisol samples analyzed were 155. The number of samples per infant were as follows: 1 sample = 21 infants (28%); 2 samples = 28 infants (37%); 3 samples = 26 infants (35%). Cortisol values were log transformed due to skewness.
3.4. Effects of stress on skin cortisol
A significant positive association was found between male gender and cortisol (p = 0.028, see Table 2), as well as male gender and stress (0.049, see Table 3).
Table 3.
ROC Analysis Results Showing Area Under the Curve for Higher Skin Cortisol by Various Durations of Stress Scores and Gestational Age
| Higher stress - Higher cortisol* |
Lower stress - Higher cortisol** |
|||||
|---|---|---|---|---|---|---|
| Duration | All | GA≥28 weeks |
GA<28 weeks |
All | GA≥28 weeks |
GA<28 weeks |
| prior 3 days | 0.54 | 0.56 | 0.27 | 0.46 | 0.44 | 0.73 |
| prior 7 days | 0.57 | 0.61 | 0.28 | 0.43 | 0.39 | 0.72 |
| prior 14 days | 0.60 | 0.64 | 0.30 | 0.40 | 0.36 | 0.70 |
| cumulative prior days | 0.58 | 0.59 | 0.33 | 0.42 | 0.41 | 0.67 |
Higher stress scores indicate higher cortisol levels;
lower stress scores indicate higher cortisol levels.
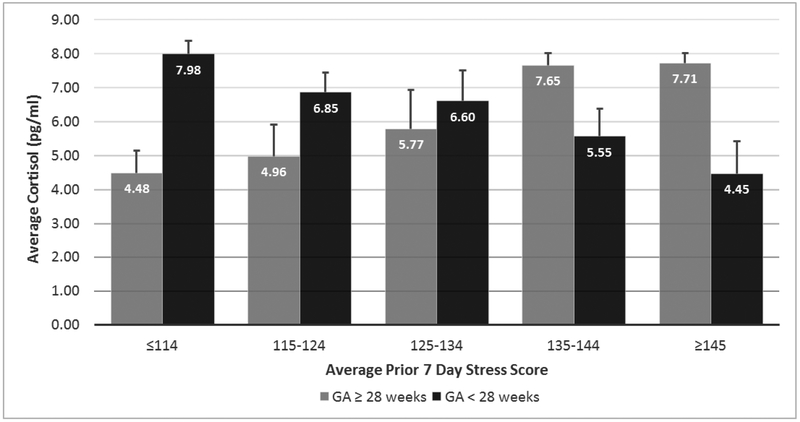
While prenatal maternal characteristics were not found to be significantly associated with infant cortisol, maternal preterm labor, premature rupture of membranes and prolonged premature rupture of membranes (PTL, PROM, PPROM) were explored as indicators of birth complications. When controlling for day of sample, gender, gestational age and birth complications, the GEE analysis between cortisol and stress revealed cortisol values to be associated through an interaction of gestational age and stress exposure during the previous 7 days (p < 0.01), see Table 4. This significant interaction indicates that the pattern of stress and cortisol was different for those infants born 28 weeks or older than infants born less than 28 weeks, see Figure 3.
Table 4.
Regression Results: Cortisol as a function of Stress
| 95% CI | |||||
|---|---|---|---|---|---|
| Model Parameter | b | SE(b) | Lower | Upper | p |
| Male | 0.2530 | 0.1247 | 0.0086 | 0.4975 | 0.04 |
| GA ≥ 28 weeks | −3.9468 | 0.8567 | −5.6259 | −2.2677 | <0.01 |
| Birth complication absent | 0.2824 | 0.1386 | 0.0108 | 0.5541 | 0.04 |
| Day of sample | 0.0691 | 0.0389 | −0.0072 | 0.1454 | 0.08 |
| Ave stress prior 7 days | −0.0002 | 0.0103 | −0.0205 | 0.0020 | 0.98 |
| Interaction: Ave stress prior 7 days * day of sample | −0.0006 | 0.0003 | −0.0012 | −0.0001 | 0.04 |
| Interaction: Ave stress prior 7 days if GA ≥ 28 weeks | 0.0289 | 0.0069 | 0.0154 | 0.0424 | <0.01 |
Figure 3.
Observed Cortisol Readings by GA Group and Prior 7 Day Stress Level. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
4. Discussion
Our exploratory study is the first to describe skin cortisol as a preterm infant biomarker of chronic stress exposure. For decades research teams have investigated normative ranges of cortisol (al Saedi et al., 1995; Heckmann et al., 1999; Ng, 2008; Ng et al., 2002), diurnal patterns (Antonini Sonir et al., 2006; Dorn et al., 2014; Ivars et al., 2017; Kidd et al., 2005) and relationships with various clinical complications (Economou et al., 1993; Helbock et al., 1993; Ng et al., 2001; Ng et al., 2004). These inquiries predominately interrogate cortisol as a means to understand preterm infant vulnerabilities from gestational age and/or medical conditions. Additionally, cortisol has been measured in preterms using various tissues such as serum (Aucott et al., 2008; Grunau et al., 2005), hair (Hoffman et al., 2016), saliva (Pawluski et al., 2012; Vittner et al., 2017) and urine (Heckmann et al., 2005; Moore, 2015). In these studies, assignment of stress acuity or chronicity is highly dependent upon the source of the cortisol.
As shown in Figure 3, for infants born 28 weeks’ gestation and older, as their stress scores increased so too did their cortisol response. This is in contrast to infants born extremely premature, or under 28 weeks’ gestation, as when their stress scores increased, their cortisol response decreased. On average, most infants cared for in the NICU tend to be ill upon admission, in turn requiring infants to be exposed to increased intervention. In subsequent weeks, interventional support often decreases as infants convalesce (D’Agata, under review; Smith et al., 2011). According to this pattern, cortisol values would be expected to be highest when stress scores are highest. In this study, for infants older than 28 weeks’ gestation, this expected pattern was observed. For infants younger than 28 weeks’ gestation though, the inverse was observed. The youngest infants did not produce a stress induced rise in cortisol.
Several hypotheses related to preterm infant physiology exist as to why this may have occurred including: a) preterm infants have limited 3B-hydroxysteroid dehydrogenase and other enzymes necessary for cortisol synthesis; b) cortisol synthesis by the adrenal cortex begins around 30 weeks; c) immaturity of the HPA and adrenal insufficiency are due to suppression of the HPA from maternal cortisol (Fernandez and Watterberg, 2009). The degree to which these physiological factors impact preterm infants contribute to inconsistent “normal” cortisol ranges for infants of different gestation ages (Heckmann et al., 1999; Ng et al., 2004). For the extremely preterm infant, the inability to adequately respond to stress, may in fact be a neuroprotective mechanism (Heckmann et al., 2005).
Several limitations should be considered with these study findings. First, not all 75 infants had skin samples available for all 3 time points and thus, missing data interfered with complete trajectory analyses. Second, due to the lack of maturation biomarkers, we lacked the ability to analyze cortisol with factors of skin maturation. Third, we lacked cortisol values during the first 2 weeks of life, which would potentially be associated with physiological response to in utero events. Considerations for future research include a larger sample size, a wide range of birth gestational age for comparison and sampling post-NICU discharge. As little concordance exists when measuring cortisol in various preterm infant tissues and results being very much dependent upon timing of postnatal age sample collection, it will be interesting to compare skin surface samples with other chronic stress tissues.
In addition to the cortisol findings, this study found that preterm infants cared for in the NICU experience an enormous amount of stress and pain exposure on a daily basis. This daily exposure does not occur just for one or two days but rather, infants are flooded with daily exposure week after week. Of concern, stress exposure was found to be significantly associated with IVH. This finding warrants further investigation to understand more about the types of stressors that may be contributing to this outcome. While neurodevelopmental response to stress exposure was not measured in these infants during their NICU stay, mounting evidence indicates preterm infants are at increased risk from early situations of high stress or toxic stress. As researchers continue to investigate the extent and implications of toxic NICU stress, skin cortisol may a method of sampling that minimally contributes to their overall stress exposure.
5. Conclusion
This study described skin cortisol as a measure of chronic stress exposure for preterm infants. For infants with appropriate skin maturation, this non-invasive sampling method provides several benefits. First, less specimen collection time is needed for clinicians and/or researchers. Second, this method may be less intrusive and disruptive for preterm infants. Lastly, if measures of chronic stress are desired, this method is reliable.
Figure 1.
Frequency of skin cortisol measurements taken by gestational age at birth. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
Highlights.
Skin sampling is a non-invasive method to measure cortisol in preterm infants.
For preterm infants >28 weeks’ gestation, skin cortisol concentrations increased as stress exposure increased.
NICU stress occurring approximately 7 days prior to skin sampling was associated with cortisol values.
Chronic stress is associated with skin cortisol.
Acknowledgement
Thank you to the parents who so generously allowed the participation of their infants in the NICU study.
NIH funding: R21NR013094 (Groer, P.I.)
Thank you for the generous support of the NICU stress collection by Little Giraffe Foundation, Chicago, IL.
Thank you to Tracy Burke and Dionna Stibila for their data collection and care of vulnerable infants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- al Saedi S, Dean H, Dent W, Cronin C, 1995. Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. The Journal of Pediatrics 126, 985–987. [DOI] [PubMed] [Google Scholar]
- Antonini Sonir RR, Jorge Salim M, Moreira Ayrton C, 2006. The emergence of salivary cortisol circadian rhythm and its relationship to sleep activity in preterm infants. Clinical Endocrinology 52, 423–426. [PubMed] [Google Scholar]
- Aucott SW, Watterberg KL, Shaffer ML, Donohue PK, Group PS, 2008. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 122, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R, Salsberry P, Ford J, 2017. Measuring stress in young children using hair cortisol: The state of the science. Biological Research For Nursing 19, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz TM, Gaehler F, Voegel CD, Hofmann M, Baumgartner MR, Kraemer T, 2018. Systematic investigations of endogenous cortisol and cortisone in nails by LC-MS/MS and correlation to hair. Anal Bioanal Chem. [DOI] [PubMed] [Google Scholar]
- Chau C, Cepeda IL, Devlin AM, Weinberg J, Grunau RE, 2015. The Val66Met brain-derived neurotrophic factor gene variant interacts with early pain exposure to predict cortisol dysregulation in 7-year-old children born very preterm: Implications for cognition. Neuroscience 342, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, 2016. Pain, gut microbiome and neurodevelopment in preterm infants, Sigma Theta Tau 27th International Nursing Research Congress, Cape Town, South Africa. [Google Scholar]
- Cong X, Wu J, Vittner D, Xu W, Hussain N, Galvin S, Fitzsimons M, McGrath JM, Henderson WA, 2017. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev 108, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata AL, Walsh S, Vittner D, Cong X, McGrath JM, Young EE, 2017. FKBP5 genotype and early life stress exposure predict neurobehavioral outcomes for preterm infants. Dev Psychobiol 59, 410–418. [DOI] [PubMed] [Google Scholar]
- D'Agata AL, Young EE, Cong X, Grasso DJ, McGrath JM, 2016. Infant Medical Trauma in the Neonatal Intensive Care Unit (IMTN): A Proposed Concept for Science and Practice. Advances in neonatal care : official journal of the National Association of Neonatal Nurses 16, 289–297. [DOI] [PubMed] [Google Scholar]
- D’Agata AL, Wu J, Sullivan MC, Groer MW, Dutra SVO, Puggioni G, under review. Understanding the Importance of NICU Stress on Health and Neurodevelopmental Outcomes. [Google Scholar]
- Dorn F, Wirth L, Gorbey S, Wege M, Zemlin M, Maier RF, Lemmer B, 2014. Influence of acoustic stimulation on the circadian and ultradian rhythm of premature infants. Chronobiology International 31, 1062–1074. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Grunau RE, Guo T, Foong J, Pearson A, Au-Young S, Lavoie R, Chakravarty MM, Chau V, Synnes A, Miller SP, 2018. Early Procedural Pain Is Associated with Regionally-Specific Alterations in Thalamic Development in Preterm Neonates. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou G, Andronikou S, Challa A, Cholevas V, Lapatsanis PD, 1993. Cortisol Secretion in Stressed Babies during the Neonatal Period. Hormone Research in Paediatrics 40, 217–221. [DOI] [PubMed] [Google Scholar]
- Evans N, Rutter N, 1986. Development of the epidermis in the newborn. Biol Neonate 49, 74–80. [DOI] [PubMed] [Google Scholar]
- Fernandez EF, Watterberg KL, 2009. Relative adrenal insufficiency in the preterm and term infant. Journal Of Perinatology 29, S44. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Provenzi L, De Carli P, Dessimone F, Sirgiovanni I, Giorda R, Cinnante C, Squarcina L, Pozzoli U, Triulzi F, Brambilla P, Borgatti R, Mosca F, Montirosso R, 2018. From early stress to 12-month development in very preterm infants: Preliminary findings on epigenetic mechanisms and brain growth. PLoS One 13, e0190602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer MW, Kane B, Williams SN, Duffy A, 2014. Relationship of PTSD Symptoms With Combat Exposure, Stress, and Inflammation in American Soldiers. Biological Research For Nursing 17, 303–310. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W, 2005. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain 113, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann M, Hartmann MF, Kampschulte B, Gack H, Bödeker R-H, Gortner L, Wudy SA, 2005. Cortisol Production Rates in Preterm Infants in Relation to Growth and Illness: A Noninvasive Prospective Study Using Gas Chromatography-Mass Spectrometry. The Journal of Clinical Endocrinology & Metabolism 90, 5737–5742. [DOI] [PubMed] [Google Scholar]
- Heckmann M, Wudy S, Haack D, Pohlandt F, 1999. Reference range for serum cortisol in well preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 81, F171–F174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbock HJ, Insoft RM, Conte FA, 1993. Glucocorticoid-Responsive Hypotension in Extremely Low Birth Weight Newborns. Pediatrics 92, 715. [PubMed] [Google Scholar]
- Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA, 1994. Adrenal steroidogenesis in very low birth weight preterm infants. The Journal of Clinical Endocrinology & Metabolism 78, 266–270. [DOI] [PubMed] [Google Scholar]
- Hoffman MC, D'Anna- Hernandez K, Benitez P, Ross Randal G, Laudenslager Mark L, 2016. Cortisol during human fetal life: Characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Developmental Psychobiology 59, 123–127. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R, 2005. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 19, 1332–1334. [DOI] [PubMed] [Google Scholar]
- Ivars K, Nelson N, Theodorsson A, Theodorsson E, Ström JO, Morelius E, 2017. Development of salivary cortisol circadian rhythm in preterm infants. PLoS ONE 12, e0182685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Baram TZ, 2009. The neuro-symphony of stress. Nat Rev Neurosci 10, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashana A, Ojaniemi M, Leskinen M, Saarela T, Hallman M, 2016. Term neonates with infection and shock display high cortisol precursors despite low levels of normal cortisol. Acta Paediatrica 105, 154–158. [DOI] [PubMed] [Google Scholar]
- Kidd S, Midgley P, Nicol M, Smith J, McIntosh N, 2005. Lack of Adult-Type Salivary Cortisol Circadian Rhythm in Hospitalized Preterm Infants. Hormone Research in Paediatrics 64, 20–27. [DOI] [PubMed] [Google Scholar]
- Lai TT, Bearer CF, 2008. Iatrogenic environmental hazards in the neonatal intensive care unit. Clin Perinatol 35, 163–181, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Ringwald C, Weber K, Engel C, Poets CF, Binder G, Bassler D, 2014. Relationship of Salivary and Plasma Cortisol Levels in Preterm Infants: Results of a Prospective Observational Study and Systematic Review of the Literature. Neonatology 105, 312–318. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Chang J, Yates C, Hall R, 2012. Challenges, guidelines, and systematic review of salivary cortisol research in preterm infants, The e-Journal of Neonatology Research. [Google Scholar]
- Moore TAS, K.K.; French J, 2015. Comparison of cortisol samples in hte first two weeks of life in preterm infants. J Pediatr Endocrinol Metab 28, 415–420. [DOI] [PubMed] [Google Scholar]
- Narendran V, Visscher MO, Abril I, Hendrix SW, Hoath SB, 2010. Biomarkers of Epidermal Innate Immunity in Premature and Full-Term Infants. Pediatric Research 67, 382. [DOI] [PubMed] [Google Scholar]
- Newnham CA, Inder TE, Milgrom J, 2009. Measuring preterm cumulative stressors within the NICU: The neonatal infant stressor scale. Early Human Development 85, 549–555. [DOI] [PubMed] [Google Scholar]
- Ng P, Lam C, Fok T, Lee C, Ma K, Chan I, Wong E, 2001. Refractory hypotension in preterm infants with adrenocortical insufficiency. Archives of Disease in Childhood. Fetal and Neonatal Edition 84, F122–F124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, Lee C, Lam C, Ma K, Fok T, Chan I, Wong E, 2004. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Archives of Disease in Childhood Fetal and Neonatal Edition 89, F119–F126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, 2008. Is There a “Normal” Range of Serum Cortisol Concentration for Preterm Infants? Pediatrics 122, 873. [DOI] [PubMed] [Google Scholar]
- Ng PC, Lam CWK, Lee CH, Ma KC, Fok TF, Chan IHS, Wong E, 2002. Reference Ranges and Factors Affecting the Human Corticotropin-Releasing Hormone Test in Preterm, Very Low Birth Weight Infants. The Journal of Clinical Endocrinology & Metabolism 87, 4621–4628. [DOI] [PubMed] [Google Scholar]
- Nonato LB, Lund CH, Kalia YN, Guy RH, 2000. Transepidermal water loss in 24 and 25 weeks gestational age infants. Acta Paediatrica 89, 747–748. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brain UM, Underhill CM, Hammond GL, Oberlander TF, 2012. Prenatal SSRI exposure alters neonatal corticosteroid binding globulin, infant cortisol levels, and emerging HPA function. Psychoneuroendocrinology 37, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Rutter N, 1996. The immature skin. European Journal of Pediatrics 155, S18–S20. [DOI] [PubMed] [Google Scholar]
- Scott SM, Watterberg KL, 1995. Effect of Gestational Age, Postnatal Age, and Illness on Plasma Cortisol Concentrations in Premature Infants. Pediatric Research 37, 112. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Garner AS, McGuinn L, Pascoe J, Wood DL, 2011. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics 129, e232–e246. [DOI] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, Inder T, 2011. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of neurology 70, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R, 2017. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 77, 261–274. [DOI] [PubMed] [Google Scholar]
- Sullivan MC, Msall ME, Miller RJ, 2012. 17-year outcome of preterm infants with diverse neonatal morbidities: Part 1--Impact on physical, neurological, and psychological health status. Journal for specialists in pediatric nursing : JSPN 17, 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MC, Winchester SB, Msall ME, 2018. Prematurity and cardiovascular risk at early adulthood. Child: care, health and development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinall J, Grunau RE, 2014. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatric research 75, 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, Walsh S, Young E, Cong X, 2017. Increase in Oxytocin From Skin-to-Skin Contact Enhances Development of Parent-Infant Relationship. Biological Research For Nursing 20, 54–62. [DOI] [PubMed] [Google Scholar]
- Xu W, Walsh S, Cong X, 2016. Development of Accumulated Pain/Stressor Scale (APSS) in NICUs: A National Survey. Pain management nursing : official journal of the American Society of Pain Management Nurses 17, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT, Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinol 3, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT, 2011. Neuroendocrinology of the skin: An overview and selective analysis. Dermatoendocrinol 3, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]