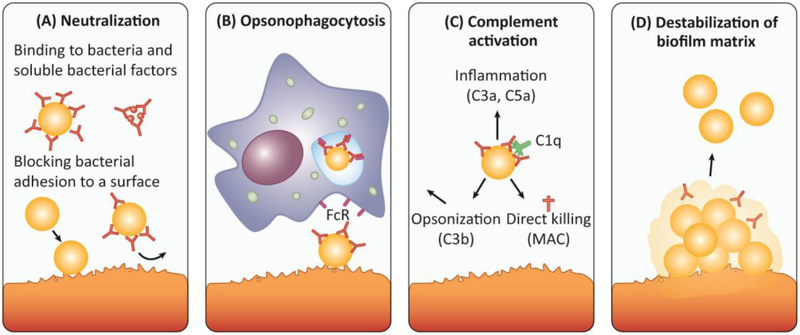
Figure 2: Antibodies can interfere with biofilm formation and promote dispersal of established biofilms by several mechanisms.
A) Secreted staphlyococcoal proteins (e.g. immune evasion molecules, toxins, exoenzymes) as well as surface proteins are involved in biofilm development and are hence potential targets for therapeutic purposes. High affinity IgA and IgG antibodies can neutralize the action of bacterial toxins and surface proteins. Moreover, antibodies can bind to bacterial adhesins (e.g. ClfA, FnBPA) and cell wall components (e.g. PNAG), thereby blocking initial attachment to host matrices and subsequent initiation of biofilm formation. B) Surface-bound antibodies (most prominently IgG) can trigger the uptake and destruction by neutrophils and macrophages expressing Fc receptors (FcR) on their surface (opsonophagocytosis). Activation of neutrophils can also trigger granule release, oxidative burst and NETosis. C) Surface-bound antibodies (IgM and IgG) trigger complement activation via the classical pathway. Following binding of C1q to the surface-bound antibody, the complement cascade is initiated resulting in the formation of the C3 convertase, which cleaves the central component of all complement pathways, C3, into C3a and C3b. C3b acts as an opsonin, enabling phagocytes that express the C3b receptor to ingest C3b-coated bacteria more easily. The soluble C3a (as well as C5a) act as chemo-attractants that recruit immune cells to the site of infection causing inflammation. C3 activation also triggers the formation of the membrane attack complex (MAC) that generates lytic pores in certain pathogens. Gram-positive bacteria, including S. aureus, are protected from MAC-dependent lysis by their thick peptidoglycan layer [132]. D) Antibodies targeting different components of the biofilm matrix, e.g. DNABII, can destabilize a biofilm matrix and thereby promote bacterial dispersal and clearance by immune cells or antibiotics.
Abbreviations: FcR (Fc receptor); MAC (membrane attack complex).