Abstract
Cohabiting children may share components of their intestinal microbiome. We evaluated whether receipt of azithromycin in one sibling confers changes to the intestinal microbiome in an untreated sibling compared with placebo in a randomized controlled trial. We found no evidence of an indirect effect of antibiotic use in cohabiting children.
Clinical Trials Registrations: NCT03187834.
The pediatric gut microbiome has been shown to be sensitive to systemic macrolide use in randomized controlled trials [1–3]. Lifetime use of macrolides has been shown to disrupt the microbiota in observational settings, and these changes may persist for many months [4, 5]. Previous work has indicated that the gut microbiome of infants is influenced by having older siblings [6–8]. Given the close physical interaction between siblings residing within a household, disruption of the microbiota in one sibling may influence the composition of the microbiome in another. As a result, antibiotic treatment of one child may alter the microbiome of another. We conducted a randomized controlled trial to assess the effect of azithromycin treatment on the composition of the gut microbiome in treated and untreated children. We hypothesized that children residing in a household with another preschool child who had been treated with azithromycin would have a significantly altered microbiome, compared with those residing in a household with a child receiving placebo.
METHODS
Setting
We conducted a randomized controlled trial in 2 rural communities of the Nouna Health and Demographic Surveillance System [9]. Detailed methods for the study have been reported elsewhere [2, 10]. The study took place in July 2017, at the beginning of the annual rainy season.
Participants and Procedures
Households were eligible for inclusion in the study if they had 2 or 3 children between the ages of 6 and 59 months according to the most recent Health and Demographic Surveillance System census. In households with 3 children, 2 randomly selected children were monitored as part of the study. Children were monitored at baseline and 5 days after the last antibiotic dose (after treatment). The study was approved by the institutional review boards at the University of California, San Francisco, and the Centre de Recherche en Santé de Nouna in Nouna, Burkina Faso. Written informed consent was obtained from each child’s caregiver.
Randomization
Households were randomized in a 1:1:1:1 fashion to placebo or 1 of 3 antibiotic arms (azithromycin, amoxicillin, or cotrimoxazole). Within each household, 1 child was randomly assigned to receive placebo, and any other children to receive the household’s randomized treatment. In households randomized to placebo, all eligible children received placebo. Of the 3 antibiotics used, only azithromycin demonstrated a significant direct change in the gut microbiome of children in the treated arm compared with placebo [2]. Therefore, for analyses of the indirect effect of antibiotics, only samples from placebo-treated children in azithromycin and placebo-treated households were processed. The randomization sequence was generated with R software (version 3.3.1; R Foundation for Statistical Computing), using a masked seed value [11].
Intervention
Study medications were prepared as a pediatric oral suspension. Azithromycin was sourced from pharmacies in Ouagadougou. Weight measurements were obtained at baseline, which were used for calculation of weight-based dosing. Azithromycin dosing was based on the lower end of the approved pediatric dosing for mild to moderate infection (a single 10-mg/kg dose on the first day, followed by 5 mg/kg once daily for 4 days). Placebo was prepared by study staff and consisted of a mixture of powdered milk, sugar, and bottled water. Study medications were prepared fresh each day and were placed in opaque orange syringes to facilitate masking. Treatment was administered from a central point in each study community, and a community mobilizer visited the homes of children participating in the study daily to instruct caregivers to bring the children for examination and treatment visits. All study medication doses were directly observed.
Sample Sequencing and Outcome Assessment
Rectal swab samples were collected at baseline and after treatment. Examiners inserted a swab 1–3 cm into the anus and rotated 360°. Swabs were placed immediately in a Stool Nucleic Acid Collection and Transport Tube containing Norgen Stool Preservative (Norgen). Samples were stored at ambient temperature in the field, and then stored at the Centre de Recherche en Santé de Nouna laboratory at −80°C until shipment to the University of California, San Francisco. Samples were shipped on ice and stored at −80°C until processing. Samples were deidentified in the field.
For library preparation and sequencing, samples were placed in a random order. DNA was extracted from the fecal samples using the Norgen stool DNA isolation kit (Norgen) per manufacturer’s instructions. Concentrations of DNA were quantified using the Qubit dsDNA HS Assay Kit (ThermoFisher Scientific) and adjusted to 15 ng/µL. The gut bacterial community was assessed by deep sequencing the V3–V4 hypervariable regions of the 16S rRNA gene. Library preparation was performed by SeqMatic per Illumina 16S metagenomic sequencing library preparation protocol. Processing of demultiplexed raw sequences were processed with QIIME software, version 1.9, which uses the Ribosomal Database Project (RDP) algorithm and the full GreenGenes 13_8 reference database to assign taxonomy to each sequencing reads. All laboratory personnel were masked to the child and household’s study arm and the time point of the sample collection (baseline or after treatment).
Sample Size Determination
The sample size was based on the primary outcome, the Simpson α-diversity between antibiotic-treated and placebo-treated children. A sample size of 30 children per arm was estimated to provide ≥80% power to detect a 1.5-unit difference in Simpson α-diversity, expressed as effective number, based on assumptions from a previous study in Niger [1].
Statistical Analysis
Baseline characteristics for the study sample were calculated as medians and interquartile ranges for continuous variables and proportions for categorical variables. We used an analysis of covariance model to assess differences in inverse Simpson and Shannon α-diversity (expressed as effective number) between children in azithromycin- and placebo-treated households. This model included a term for the household randomization arm (azithromycin or placebo) and the baseline diversity measure. We calculated the difference in Simpson and Shannon diversity before versus after treatment and used a linear regression model to assess differences in the change between arms. All P values were calculated using a Monte Carlo permutation test with 9999 replications, and differences were considered statistically significant at P < .05. All analyses were conducted using R software, version 3.5.1.
RESULTS
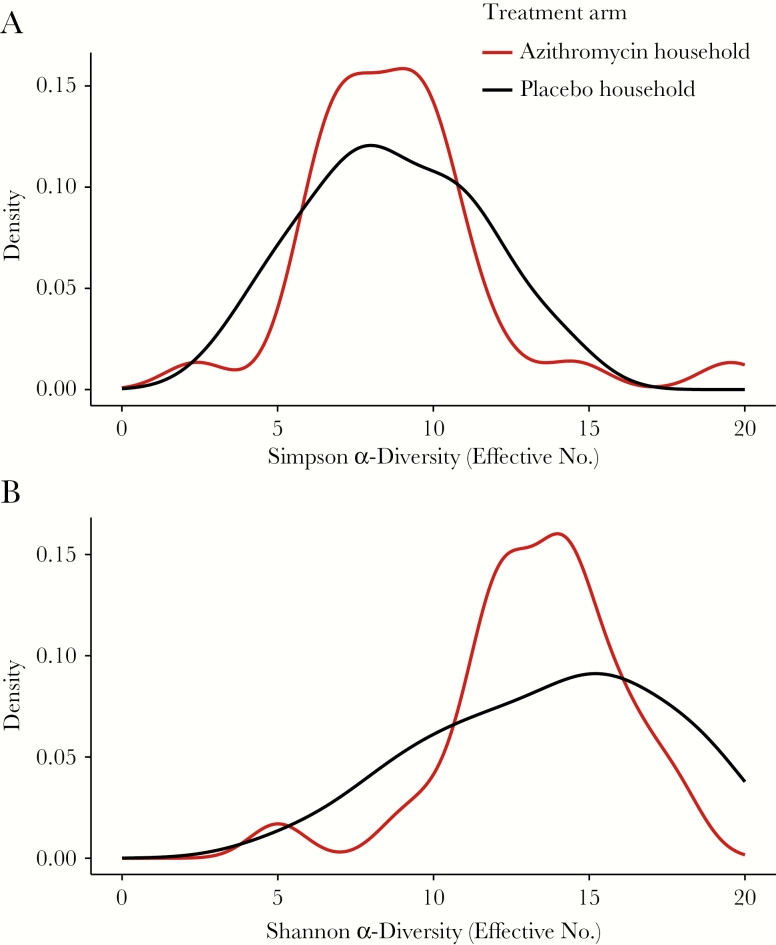
A total of 62 untreated children in 62 households (31 azithromycin, 31 placebo) were included in this analysis (Supplementary Figure 1). Baseline characteristics were similar between study arms (Supplementary Table 1). At baseline, the mean inverse Simpson α-diversity was 10.8 (95% confidence interval [CI], 9.6–11.9) in azithromycin households and 8.8 (7.7–10.0) in placebo households. The mean inverse Shannon α-diversity was 17.1 (95% CI, 15.6–18.7) in azithromycin households and 15.2 (13.5–16.8) in placebo households. Five days after the last antibiotic dose, the mean inverse Simpson α-diversity was 9.3 (95% CI, 7.7–10.6) in azithromycin households and 8.7 (7.6–9.8) in placebo households (Figure 1A). The mean Shannon α-diversity was 15.1 (95% CI, 13.1–17.2) in azithromycin households and 14.3 (12.6–15.9) in placebo households (Figure 1B). There was no difference across study arms in a linear regression model in the change from before and after treatment for Simpson (P = .21) or Shannon (P = .47) α-diversities. Similarly, there was no difference in Simpson or Shannon α-diversity in an analysis of covariance model, which included a term for baseline diversity (P = .73 and P = .75, respectively).
Figure 1.
Posttreatment Simpson (A) and Shannon (B) diversity of gut bacteria in untreated siblings of children treated with azithromycin (red line) or placebo (black line).
DISCUSSION
In a randomized controlled trial in rural Burkina Faso, we found no evidence that treatment of a child with a 5-day course of azithromycin affected the diversity of the intestinal microbiome in untreated siblings. Antibiotic treatment has been shown to confer herdlike effects in untreated individuals at the community level with mass azithromycin distribution [12] and in hospitalized patients who occupy the bed of a patient who previously received antibiotics [13]. Findings of a US study of intestinal microbiome in close contacts of individuals taking antibiotics suggested that antibiotic treatment could alter the microbiome of untreated household contacts [14]. Although we hypothesized that a similar effect would be seen in untreated children cohabiting with antibiotic-treated children, our results suggest that treatment of a sibling may not alter the microbiome of an untreated child.
This study was powered for the primary outcome, the direct effect of antibiotic use on diversity in the treated child’s microbiome. Any alterations in the microbiome of an untreated child would probably be smaller than direct effects, and this study may have been underpowered to detect an indirect effect. Children in this study were followed up for only for 5 days after the sibling’s antibiotic treatment. Although we would not necessarily expect longer-term disruption in the composition of the microbiome in the absence of any shorter-term effect in treated individuals, indirect effects of antibiotics may take longer to develop in untreated individuals, because presumably any indirect effect would require repeated contact between children. Future studies could consider longer-term evaluation of the indirect effect of antibiotics in cohabiting children.
Although the full impact of antibiotic use on the composition of the gut microbiome in children remains unknown, results of the current study do not provide evidence that azithromycin consumption by a cohabiting sibling affects gut microbial diversity in untreated children in the short term, despite strong evidence of a direct effect on diversity [2].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Disclaimer. The funders did not play a role in study design, analysis, interpretation of results, or decision to publish.
Financial support. This work was supported in part by Research to Prevent Blindness (career development awards to C. E. O. and T. D.) and the National Eye Institute, National Institutes of Health (grant K08EY026986 to T. D.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Doan T, Arzika A, Ray KJ, et al. . Gut microbial diversity in antibiotic-naïve children after systemic antibiotic exposure: a randomized controlled trial. Clin Infect Dis 2017; 64:1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oldenburg CE, Sie A, Coulibaly B, et al. . Effect of commonly-used pediatric antibiotics on gut microbial diversity in preschool children in Burkina Faso: a randomized clinical trial. Open Forum Infect Dis 2018; 5:ofy289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doan T, Hinterwirth A, Arzika AM, et al. . Mass azithromycin distribution and community microbiome: a cluster-randomized trial. Open Forum Infect Dis 2018; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korpela K, Salonen A, Virta LJ, et al. . Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 2016; 7:10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korpela K, Zijlmans MAC, Kuitunen M, et al. . Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017; 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin R, Makino H, Cetinyurek Yavuz A, et al. . Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 2016; 11:e0158498-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laursen MF, Zachariassen G, Bahl MI, et al. . Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol 2015; 15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azad MB, Konya TB, Maughan H, et al. . Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy, Asthma, Clin Immunol 2013; 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sie A, Louis VR, Gbangou A, et al. . The Health and Demographic Surveillance System (HDSS) in Nouna, Burkina Faso, 1993–2007. Glob Health Action 2010; 3:5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sie A, Dah C, Ouermi L, et al. . Effect of antibiotics on short-term growth among children in Burkina Faso: a randomized trial. Am J Trop Med Hyg 2018; 99:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porco TC, Stoller NE, Keenan JD, Bailey RL, Lietman TM. Public key cryptography for quality assurance in randomization for clinical trials. Contemp Clin Trials 2015; 42:167–8. [DOI] [PubMed] [Google Scholar]
- 12. House JI, Ayele B, Porco TC, et al. . Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet 2009; 373:1111–8. [DOI] [PubMed] [Google Scholar]
- 13. Freedberg DE, Salmasian H, Cohen B, et al. . Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016; 176:1801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abeles SR, Jones MB, Santiago-Rodriguez TM, et al. . Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome 2016; 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.