Abstract
Continuous fermentation of dilute acid-pretreated de-oiled rice bran (DRB) to butanol by the Clostridium acetobutylicum YM1 strain was investigated. Pretreatment of DRB with dilute sulfuric acid (1%) resulted in the production of 42.12 g/L total sugars, including 25.57 g/L glucose, 15.1 g/L xylose and 1.46 g/L cellobiose. Pretreated-DRB (SADRB) was used as a fermentation medium at various dilution rates, and a dilution rate of 0.02 h−1 was optimal for solvent production, in which 11.18 g/L of total solvent was produced (acetone 4.37 g/L, butanol 5.89 g/L and ethanol 0.92 g/L). Detoxification of SADRB with activated charcoal resulted in the high removal of fermentation inhibitory compounds. Fermentation of detoxified-SADRB in continuous fermentation with a dilution rate of 0.02 h−1 achieved higher concentrations of solvent (12.42 g/L) and butanol (6.87 g/L), respectively, with a solvent productivity of 0.248 g/L.h. This study showed that the solvent concentration and productivity in continuous fermentation from SADRB was higher than that obtained from batch culture fermentation. This study also provides an economic assessment for butanol production in continuous fermentation process from DRB to validate the commercial viability of this process.
Introduction
The worldwide energy demand is continuously increasing over time due to the expected decline of petrol and due to environmental issues that are related to the use of petrol as a source of energy1. Petroleum oil is a non-renewable resource and is going to be depleted soon. Accordingly, it is necessary to find renewable alternative sources of fuel that can substitute for oil and are environmentally friendly. One of the best liquid biofuels that can substitute for gasoline is butanol, which has similar properties as gasoline.
Butanol is produced biologically by acetone-butanol-ethanol (ABE) fermentation using solventogenic Clostridium species. Clostridium acetobutylicum YM1 is a solvent-producing strain that was isolated from local agricultural soil in Malaysia and has been used for butanol and hydrogen production2,3.
The substrate cost, microbial strain performance, fermentation process mode and recovery process significantly affect the economics of butanol production. The use of low cost and sustainable feedstocks for butanol production can minimize the cost of this process4. As reported in the literature, the most influential factor in ABE fermentation is the cost of the substrate, which constitutes approximately 60% of the total process cost5. Hence, exploring less expensive substrates for ABE fermentation is essential for making ABE fermentation economically viable. Agricultural biomass residues are a suitable alternative because of its low price feedstocks. However, prior to utilizing lignocellulosic feedstocks, they require pretreatment and saccharification.
Developing excellent strains that are resistant to butanol toxicity and hyper-butanol producing is an ideal idea for improving butanol fermentation, but it still needs more efforts. Some Clostridium strains have been engineered using systematic or mutagenesis approaches to improve butanol productivity and overcome the butanol toxicity. Earlier, mutagenesis and genetic manipulation methods such as homologous recombination and antisense RNA were used to understand gene functions and enhance butanol production. A new technique called CRISPR-Cas provided large-scale genome editing of Clostridium over than mutagenesis and genetic manipulation techniques. CRISPR-Cas-based editing tool kits is also a promising biotechnology which can be used for efficient Clostridium cell engineering for improving butanol production6. A comprehensive review on recent strategies for strain development and advanced downstream process techniques for butanol production by Clostridium acetobutylicum is detailed by Xue et al.6.
Batch culture fermentation of butanol production by ABE fermentation is the most practiced fermentation for butanol production, while in industrial large scale of butanol production, the continuous fermentation mode is more productive than the batch fermentation mode. There are many shortcomings in the batch fermentation operation of butanol including the accumulation of butanol which halts the fermentation due to the toxicity, the period required for medium preparation and bioreactor sterilization during which processes are ceased, considerable down time and low yield and productivity.
Butanol production by continuous fermentation prevents the accumulation of butanol and then eliminates the cytotoxicity of butanol. The feeding of fresh medium and the elimination of product accumulation is a useful method that can keep the operation going at a steady rate which results into significant enhancement in the butanol yield and productivity. Continuous ABE fermentation has several benefits compared to batch fermentation, including higher productivity, less product inhibition, and less downtime, while there are some difficulties associated with continuous ABE fermentation, such as the two-phase nature of ABE fermentation (acidogenesis and solventogenesis), phage contamination and flocculation of bacterial growth, which can make steady state fermentation unstable. The solvent productivity in the batch ABE fermentation process is usually low while the solvent productivity in continuous fermentation is greater, which makes continuous ABE fermentation more attractive for commercial industrial ABE production. In continuous ABE fermentation, glucose or corn starch are mainly consumed as the major feedstock7.
Cell immobilization of clostridia is an efficient approach to obtain a high productive continuous fermentation system for butanol production. Cell immobilization protects the cells from butanol toxicity and prevents them from bleeding during continuous fermentation. Butanol production using an immobilized cell of Clostridium sp. in continuous fermentation systems improved the fermentation productivity and stability8. Due to the biphasic of ABE fermentation, it was found that a single chemostat bioreactor is not applicable to operate continuous fermentation for high productivity of butanol and tanks-in-series systems were suggested as an option for high efficient system using sustainable feedstocks and efficient microbial strains9.
Integrated butanol recovery techniques such as gas stripping, pervaporation, liquid-liquid extraction and adsorption could remove butanol simultaneously during the ABE fermentation, reduce the butanol toxicity and subsequently increase fermentation productivity10. Advanced integrated recovery techniques for in situ butanol separation with using an engineered microbial strain could also improve the efficiency and stability of butanol production, which was proposed to make this process viable economically6. The conventional recovery technique for butanol is distillation which is characterized to be high-energy consumption and not economically competitive whereas in situ butanol recovery technologies are energy-saving and can be applied during the fermentation to reduce the product toxicity and improve butanol productivity10,11.
Rice is the staple food of more than 3.5 billion people and the worldwide production of rice is expected to reach 480.1 million metric tons in 201712. Rice bran is a residual waste of the rice processing industry that accounts for approximately 10% of rice production. Rice bran is rich in oil and the waste of oil from extraction is called de-oiled rice bran (DRB). DRB is available, is inexpensive, contains large amounts of carbohydrates and has limited application as an animal feed. Therefore, DRB is a potential substrate for an economically viable butanol production process13.
Prior to the bioconversion of agricultural residues to butanol by Clostridium, a pretreatment/hydrolysis step is required to release fermentable sugars, which can then be utilized by Clostridium strains for butanol production14. Various pretreatment approaches, including physical and chemical methods or a combination of the two methods, have been applied on agricultural biomass to produce fermentable sugars15. The most common pretreatment method used for the pretreatment of agricultural biomass is dilute sulfuric acid, in which the agricultural biomass is exposed to high temperature and dilute sulfuric acid.
During the pretreatment process of lignocellulosic biomass, a number of inhibitor compounds are usually produced as a result of extreme degradation. These fermentation inhibitory compounds are including furfural, hydroxymethylfurfural (HMF), acetic, formic, ρ- coumaric, ferulic, levulinic, glucuronic acids, and phenolic compounds that inhibit bacterial growth and then negatively affect the butanol fermentation efficiency16. Many methods have been applied to remove or decrease these inhibitory compounds, including the use of adsorbent resin and activated charcoal, the dilution of hydrolysate, overliming and the development of tolerant microbial strains13,17,18.
In the current study, continuous fermentation for butanol production was explored using dilute sulfuric acid pretreated-DRB as a fermentation medium by C. acetobutylicum YM1. The butanol production from SADRB by the strain YM1 in a continuous fermentation process was carried out at different dilution rates and using non-detoxified and detoxified SADRB hydrolysate.
Results and Discussion
Batch fermentation of SADRB
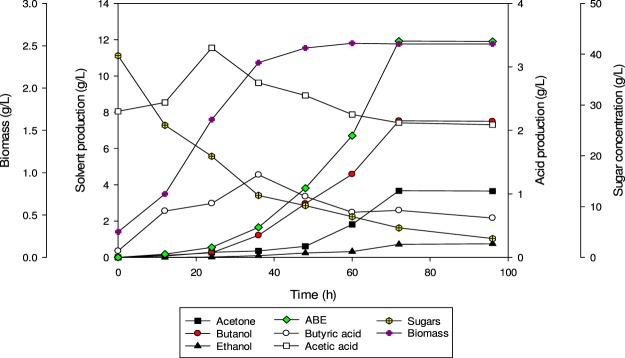
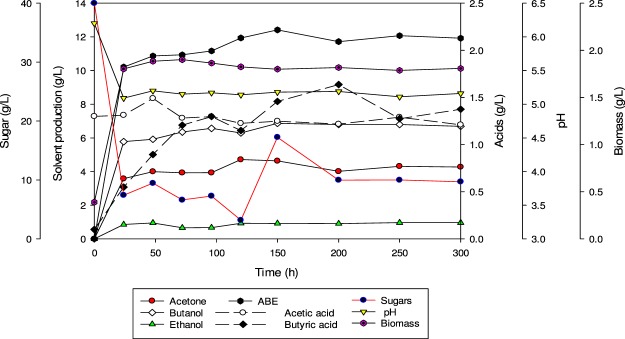
Batch fermentation experiments with SADRB using C. acetobutylicum YM1 were conducted as a control to compare the fermentation performance. The initial concentration of total sugars was 39.7 g/L and the fermentation was started by inoculating the medium with 10% (v/v) fresh inoculum of C. acetobutylicum YM1. In this experiment, the maximum production of butanol and ABE was obtained after 72 h, at 7.53 g/L and 11.92 g/L, respectively. Acetone and ethanol concentrations also reached their maximum at 72 h (Fig. 1). The sugar was mostly consumed, and only 8 g/L was left after 72 h of fermentation time. The ratio of butanol to acetone was 2: 1, which is a typical ratio in ABE fermentation as reported in the literature19. The yields of butanol and ABE were 0.25 g/g and 0.38 g/g, respectively, and the production rates of butanol and ABE were 0.105 g/L.h and 0.166 g/L.h, respectively. The butyric acid concentration reached a maximum value (1.3 g/L) at 36 h, while acetic acid was maximized at 24 h. Then, both acids were utilized during the solventogenic phase, with final concentrations of butyric and acetic acids of 0.74 g/L and 2.12 g/L, respectively, after 72 h of fermentation. Butyric acid utilization was clearly associated with butanol triggering, which is initiated once the bacterial growth reaches a stationary phase as a secondary metabolite (Fig. 1). In comparison, 9.66 g/L of total ABE with 6.75 g/L of butanol was produced from fermentation with SADRB (33.4 g/L sugar) by C. saccharoperbutylacetonicum N1-4, with an ABE yield and productivity of 0.35 g/g and 0.081 g/L.h, respectively13.
Figure 1.
Butanol production in batch culture fermentation of SADRB by C. acetobutylicum YM1.
Batch fermentation of detoxified SADRB
SADRB hydrolysate was detoxified by activated charcoal to reduce or remove inhibitory compounds from the hydrolysate, including furfural, HMF, acetic acid, formic acid and levulinic acid. The concentrations of fermentation inhibitors before detoxification and after detoxification by charcoal are listed in Table 1.
Table 1.
Characteristic of non-detoxified SADRB and detoxified SADRB by activated charcoal.
| Fermentation Inhibitors | Non-detoxified SADRB | Detoxified SADRB | Reduction (%) |
|---|---|---|---|
| Total Sugars (g/L) | 42.12 | 40.17 | 4.63 |
| Furfural (g/L) | 0.33 | 0.0012 | 99.64 |
| HMF (g/L) | 0.46 | 0.0014 | 99.69 |
| Acetic acid (g/L) | 2.70 | 1.30 | 51. 85 |
| Formic acid (g/L) | 0.69 | 0.10 | 85.51 |
| Levulinic acid (g/L) | 1.21 | 0.59 | 50.85 |
Activated charcoal showed a high potential for reducing fermentation inhibitors from SADRB hydrolysate, and a nonsignificant concentration of sugars was reduced (4.63%), as shown in Table 1. The removal efficiencies of furfural, 5-HMF, acetic acid, formic acid and levulinic acid were 99.64, 99.69, 51.85, 85.51 and 50.85%, respectively.
Activated charcoal can be regenerated after being used in detoxification to reduce the cost of the process. The most common method used for the regeneration of activated charcoal is thermal regeneration. Moreover, gasification by air, CO2 or nitrogen, heating by microwave, pyrolysis and wet oxidation techniques have also been applied for the regeneration of activated charcoal20–22. Regeneration of activated charcoal had some benefits such as reducing the use of the coal and the natural resources, reducing the pollution caused by the waste of the used activated charcoal. In addition, the energy required to regenerate activated charcoal is less than what is needed to produce new activated charcoal22. However, some studies have reported poor regeneration efficiency of activated charcoal due to the oligomerization of phenolic compounds. The irreversible adsorption of phenolic compounds onto activated charcoal represents a major problem which reduces the lifetime of the activated charcoal usage, increases the operation cost and contributes to the pollution due to the disposal of the used activated charcoal. Though, phenol-loaded activated charcoal was regenerated by electrochemical regeneration technique with 80% of regeneration efficiencies23. Moreover, a special activated charcoal was developed for hampering oligomerization of phenolic compounds on its surface by controlling the activation process for obtaining high microporosity24.
It was noticeable that the concentration of acetic acid in the SADRB hydrolysate was high (2.5 ± 0.2 g/L). Acetic acid is released from the hydrolysis of hemicellulosic materials that contain many acetyl groups. It was reported that acetic acid has an inhibitory impact on the biomass concentration of C. acetobutylicum at high concentrations (0.19 M)25,26. Therefore, as a detoxification response, C. acetobutylicum converts acetic acid to acetone in the solventogenic phase through an enzymatic system27.
Detoxification of SADRB hydrolysate by activated charcoal resulted in a significant decrease in the acetic acid portion (51.85%). Moreover, dilute sulfuric acid pretreatment of DRB released 0.69 g/L of formic acid. Previously, it was found that the presence of formic acid in the fermentation medium led to a high reduction of butanol production by C. acetobutylicum, that ABE production was reduced to 77% in the presence of 1 g/L formic acid and that there was a 25% reduction upon addition of 0.4 g/L formic acid26. Furthermore, Wang et al. reported that the addition of 0.046 g/L of formic acid to corn mash medium with C. acetobutylicum caused an acid crash during ABE fermentation and this effect of formic acid on ABE fermentation might be mediated through oxidative stress28. In a toxicity test, we found that the addition of 1 g/L formic acid to the medium of C. acetobutylicum YM1 resulted in total growth inhibition (data will be published elsewhere). Interestingly, detoxification of SADRB hydrolysate by activated charcoal decreased the concentration of formic acid to 0.1 g/L (85.51%), which showed the considerable removal efficiency of charcoal.
In the literature, it was found that detoxification of hardwood Kraft black liquor hydrolysate using activated charcoal could recover 99–100% of xylose29, which means that negligible xylose was lost during detoxification, which is in agreement with our results. Moreover, Mussatto and Roberto30 and Kamal et al.31 reported that activated charcoal has a high ability to adsorb fermentation inhibitor compounds with less reduction of the sugar concentration30,31. In a study conducted by Guo et al., it was found that detoxification of spruce hydrolysate by activated charcoal resulted in the removal of 94% furfural and HMF32.
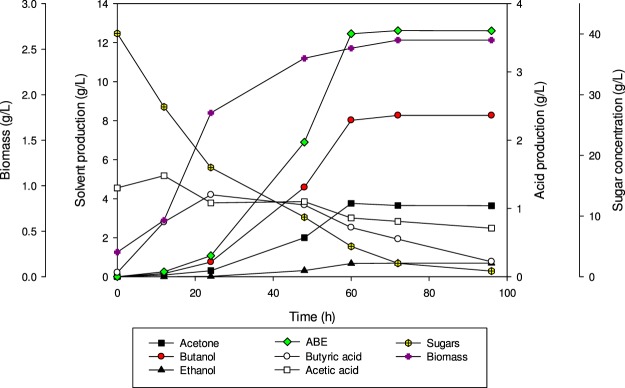
Batch fermentation of detoxified SADRB was used for butanol production by C. acetobutylicum YM1. The starting sugar concentration was 40.1 g/L and only 0.92 g/L of residual sugar remained in the culture after 96 h of fermentation. The maximum total ABE, butanol, acetone and ethanol obtained after 72 h of batch fermentation was 12.62, 8.27, 3.65 and 0.7 g/L, respectively (Fig. 2). The ABE and butanol produced from detoxified SADRB were higher than that produced when non-detoxified SADRB was used in batch fermentation with C. acetobutylicum YM1 under similar conditions. The productivities of butanol (0.115 g/L.h) and total ABE (0.175 g/L.h) in this experiment were also higher than when non-detoxified of SADRB was employed. The results showed that detoxified SADRB produced higher concentrations of solvents compared to that produced from non-detoxified SADRB.
Figure 2.
Butanol production in batch culture fermentation of detoxified SADRB by C. acetobutylicum YM1.
Continuous fermentation of glucose
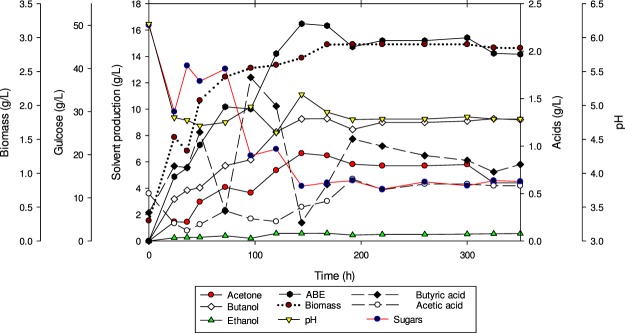
Continuous fermentation using 50 g/L of glucose at a dilution rate of 0.05 h−1 was conducted as a control test. Figure 3 represents the continuous fermentation profile of butanol production by C. acetobutylicum YM1. The steady state was reached after 120 h of fermentation and continued afterward. The glucose consumption was maintained between 12–14 g/L under steady-state fermentation when the dilution rate was 0.05 h−1.
Figure 3.
Profiles of continuous fermentation for butanol production from glucose (5%) using C. acetobutylicum YM1 with a dilution rate of 0.05 h−1.
The average total ABE production at the steady state was 16.51 g/L with butanol, acetone and ethanol concentrations of 9.28, 6.65 and 0.58 g/L, respectively. The biomass concentration was also kept constant in the stationary phase, and no decline of biomass concentration was observed during 350 h of continuous fermentation (Fig. 3). The butyric acid concentration increased up to 1.14 g/L after 48 h and after that, the concentration was decreased and kept constant, with an average concentration of 0.8 ± 0.2 g/L, while the concentration of acetic acid was also constant at steady state with a concentration of 0.5 ± 0.1 g/L. Culture pH was not controlled in this study and was constant after 24 h of fermentation at pH 4.8 ± 0.1 during the whole fermentation time. In this experiment, the productivities of total solvent and butanol were 0.823 and 0.464 g/L.h, respectively. The yields of ABE and butanol in the continuous fermentation of butanol from glucose by C. acetobutylicum YM1 were 0.48 g/g and 0.25 g/g, respectively.
In comparison, the ABE productivity in continuous fermentation with 5% (w/v) glucose by C. acetobutylicum YM1 was higher than that found when batch fermentation was performed using C. acetobutylicum YM1 in 5% (w/v) glucose, representing an 87.1% increase. Batch fermentation of 5% glucose resulted in 12.72 g/L total solvents in 120 h, including 3.09 g/L acetone, 9.48 g/L butanol and 0.16 g/L ethanol. Therefore, the results showed that continuous fermentation of 5% glucose by C. acetobutylicum YM1 was superior in solvent production and productivity compared to batch culture fermentation.
Liew et al., reported lower solvent production (9.1 g/L) compared to our study when they operated a continuous fermentation by Clostridium saccharobutylicum DSM 13864 at a dilution rate of 0.05 h−1 33. In addition, the solvent productivity (0.823 g/L.h) obtained in this study was higher compared to that reported by Liew et al., at 0.46 g/L.h33.
Continuous fermentation of SADRB
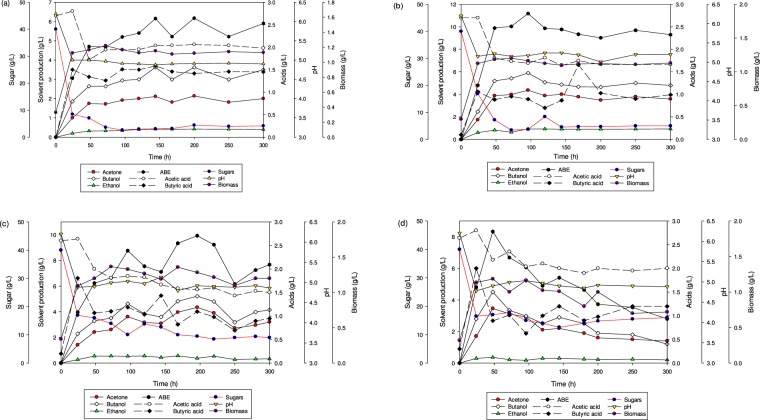
SADRB was used as a fermentation medium for the continuous fermentation of butanol by C. acetobutylicum YM1. Various dilution rates were applied: 0.01, 0.02, 0.03 and 0.05 h−1. Profiles of continuous fermentation performance, solvent concentrations, acids, biomass concentration, pH and sugar utilization are presented in Fig. 4(a–d). It was noticeable that the steady state occurred when continuous fermentation of SADRB was performed at dilution rates of 0.01, 0.02 and 0.03 h−1 and could be stably maintained during 300 h of continuous fermentation. While with a dilution rate of 0.05 h−1 the solvent production reached a maximum after 48 h of 8.33 g/L, it started decreasing along with fermentation time thereafter (Fig. 4d). Ni et al., found that the continuous fermentation of butanol by Clostridium saccharobutylicum enters a steady state when a stable solvent concentration is obtained in the fermentation period between 100–200 h34.
Figure 4.
Continuous fermentation profiles of butanol production from SADRB using C. acetobutylicum YM1; (a) at a dilution rate of 0.01 h−1, (b) at a dilution rate of 0.02 h−1, (c) at a dilution rate of 0.03 h−1 and (d) at a dilution rate of 0.05 h−1.
Concentrations of ABE, butanol, acetone, and ethanol, as well as yields and productivities of ABE and butanol, are listed in Table 2 for all tested dilution rates using SADRB. Among all the tested dilution rates, a dilution rate of 0.02 h−1 produced the highest production rates of ABE and butanol, at 11.18 and 5.89 g/L, respectively.
Table 2.
Performance of batch and continuous fermentation of butanol production by C. acetobutylicum YM1.
| Parameters | Batch fermentation | Continuous fermentation | ||||||
|---|---|---|---|---|---|---|---|---|
| SADRB | Detoxified SADRB | Glucose (5%) | Non detoxified SADRB | Detoxified SADRB | ||||
| Dilution rate (h−1) | — | — | 0.05 | 0.01 | 0.02 | 0.03 | 0.05 | 0.02 |
| ABE (g/L) | 11.92 | 12.62 | 16.51 | 6.17 | 11.18 | 9.93 | 8.33 | 12.42 |
| Butanol (g/L) | 7.53 | 8.27 | 9.28 | 3.63 | 5.89 | 5.21 | 4.51 | 6.87 |
| Acetone (g/L) | 3.67 | 3.65 | 6.65 | 2.14 | 4.37 | 4.34 | 3.46 | 4.63 |
| Ethanol (g/L) | 0.72 | 0.7 | 0.58 | 0.42 | 0.92 | 0.57 | 0.36 | 0.92 |
| ABE productivity (g/L.h) | 0.166 | 0.175 | 0.823 | 0.062 | 0.224 | 0.298 | 0.417 | 0.248 |
| Butanol productivity (g/L.h) | 0.105 | 0.115 | 0.464 | 0.036 | 0.118 | 0.156 | 0.226 | 0.136 |
| B:A ratio | 2.05 | 2.27 | 1.4 | 1.7 | 1.35 | 1.2 | 1.3 | 1.5 |
| ABE yield (g/g) | 0.38 | 0.32 | 0.48 | 0.17 | 0.34 | 0.28 | 0.29 | 0.43 |
| Butanol yield (g/g) | 0.25 | 0.21 | 0.27 | 0.10 | 0.18 | 0.15 | 0.16 | 0.24 |
In continuous fermentation, it was found that high solvent productivity could be obtained at low dilution rates35. Higher dilution rates were reported to be more suitable for bacterial biomass concentrations, while lower dilution rates were found to be optimal for ABE and butanol production in single-stage continuous fermentation33. In our study, we found that increasing the dilution rate to more than 0.02 h−1 resulted in lower concentrations of solvent and butanol, which was in agreement with that reported by Godin and Engasser35.
Continuous fermentation of SADRB at a dilution rate of 0.05 h−1 showed poor performance and the steady-state stage was shorter than that observed with other employed dilution rates. The bacterial biomass concentration and butanol production declined along with the fermentation time (Fig. 4d). The instability of the fermentation at this dilution rate can be attributed to a decline in bacterial biomass concentration, which is also likely due to the destruction of bacterial cells during the fermentation. The maximum ABE and butanol concentrations obtained at 48 h were 8.33 g/L and 4.51 g/L, respectively, and the concentrations of ABE and butanol decreased after 48 h. Sugars were consumed efficiently at the first 48 h and after that, an average of 15 ± 1 g/L sugars remained (Fig. 4d). Accordingly, the suitable dilution rate that maximized ABE and butanol production from SADRB hydrolysate in single-stage continuous fermentation was 0.02 h−1.
In continuous fermentation of SADRB, it was observed that increasing the dilution rates led to a decrease in the butanol to acetone ratio (B:A), and the highest ratio of B:A was obtained at a dilution rate 0.01 h−1 (Table 2). The decrease of B:A at higher dilution rates can be attributed to the fact that at high dilution rates the dominant bacterial cells are in log phase where more acids are produced, and reutilization of these acids is associated with acetone production36,37. Similar results were found by Liew et al., when they increased the dilution rate of continuous fermentation of sago starch from 0.03 to 0.22 h−1, and a decrease in B:A ratios was observed, with the highest B:A obtained being 1.5533.
Continuous fermentation of detoxified-SADRB
Detoxified SADRB was employed for butanol fermentation in the continuous mode with the strain C. acetobutylicum YM1 at a dilution rate of 0.02 h−1. The detoxified SADRB contained 40.17 g/L total sugars, and the sugars detected were 24.14 g/L glucose (60.87%), 14.58 g/L xylose (35.59%) and 1.45 g/L cellobiose (3.54%).
A continuous fermentation time-course of detoxified SADRB is shown in Fig. 5. It can be seen that the butanol concentration reached a high concentration after 24 h and continued at a steady state until the fermentation was stopped after 300 h. The bacterial biomass concentration was constant in stationary phase and no decrease in biomass concentration was observed during the fermentation. In addition, the bacterial biomass concentration in the detoxified SADRB culture was higher compared to that found when non-detoxified SADRB was employed (Figs 4b and 5). The results demonstrated that detoxification of SADRB by charcoal reduced fermentation inhibitors and then allowed the cells to grow better, which indicated that detoxification is essential for enhanced ABE fermentation performance. Continuous fermentation of detoxified SADRB produced a total of 12.42 g/L ABE containing 6.87 g/L butanol, 4.63 g/L acetone and 0.92 g/L ethanol and giving an ABE yield and productivity of 0.43 g/g and 0.428 g/L.h, respectively.
Figure 5.
Profiles of single stage continuous fermentation for butanol production from detoxified SADRB hydrolysate using C. acetobutylicum YM1 at a dilution rate of 0.02 h−1.
Compared to non-detoxified SADRB, in which lower ABE and butanol were produced, detoxified SADRB resulted in higher concentrations of ABE and butanol, which most likely was due to the removal of inhibitory compounds that cause cell inhibition and subsequently lower ABE fermentation efficiency. Detoxification by activated charcoal has been used previously for the removal of fermentation toxic compounds from different biomass hydrolysates38–41. Activated charcoal showed that it has good efficiency for the removal of fermentation inhibitors and it can be regenerated after detoxification40.
Table 3 compares continuous butanol production from SADRB and glucose in this study and the results reported in the literature. The results of this study showed that C. acetobutylicum YM1 has high efficiency for butanol production in continuous fermentation from both substrates, glucose and SADRB hydrolysate.
Table 3.
Comparison of continuous fermentation of butanol from different substrates.
| Substrate | Strain | Dilution rate (h−1) | ABE (g/L) | Butanol (g/L) | ABE productivity (g/L.h) | Reference |
|---|---|---|---|---|---|---|
| Corn stover | C. saccharobutylicum DSM 13864 | 0.15 | 11.43 | 7.81 | 0.429 | 34 |
| Cane molasses | C. saccharobutylicum DSM 13864 | 0.1 | 11.74 | 7.18 | 0.294 | 34 |
| Sago starch | C. saccharobutylicum DSM 13864 | 0.05 | 9.10 | 5.19 | 0.455 | 33 |
| Corn starch | C. beijerinckii BA101 | 0.02 | 7.20 | — | 0.144 | 43 |
| De-fibrated-sweet potato-slurry | C. acetobutylicum P-262 | 0.129 | 7.73 | 5.52 | 1.00 | 53 |
| Glucose (6%) | Immobilized C. acetobutylicum | 0.05 | 11.74 | 7.80 | 0.630 | 42 |
| Glucose (5%) | C. acetobutylicum YM1 | 0.05 | 16.51 | 9.28 | 0.823 | This study |
| Deoiled rice bran | C. acetobutylicum YM1 | 0.02 | 12.42 | 6.87 | 0.248 | This study |
Based on the data presented in Table 3, ABE and butanol produced by C. acetobutylicum YM1 in continuous fermentation using 5% glucose was satisfactorily higher than ABE and butanol as reported by Dolejs et al. in the continuous fermentation of immobilized C. acetobutylicum fed with a glucose concentration of 60 g/L under the same dilution rate of 0.05 h−1 42. Additionally, a higher ABE productivity of 0.823 g/L.h was obtained in this study compared to the 0.63 g/L.h ABE productivity found by Dolejs et al.42.
Moreover, continuous fermentation of detoxified SADRB by C. acetobutylicum YM1 at a dilution rate of 0.02 h−1 resulted in the production of 12.42 ABE and 6.87 g/L butanol with a productivity of 0.248 g/L.h, which was higher than that found by Ezeji et al.43 when corn starch was used as a substrate in continuous fermentation by C. beijerinckii BA101 (Table 3). Continuous fermentation of dilute acid pretreated-corn stover at a dilution rate of 0.15 h−1 conducted by Ni et al.34 showed lower concentration of ABE but higher ABE productivity compared to that obtained in our study using SADRB hydrolysate.
According to the results that were summarized in Table 2, it can be seen that the productivities and yields of ABE and butanol in continuous fermentation were higher than that obtained under the batch fermentation process. The ABE and butanol productivities gained in continuous fermentation using glucose as a substrate were approximately 5 times higher than that obtained from batch fermentation of SADRB. In a similar trend, the ABE and butanol productivities found when detoxified SADRB was consumed in continuous fermentation were 2.6 times and 1.3 times higher, respectively, compared to that found using batch fermentation.
The continuous fermentation process is preferable in industry due to the high productivity and lower preparation time44. For the further improvement of butanol fermentation performance, applying in situ continuous fermentation with a product recovery approach has been suggested10,45,46. Applying process integration systems for butanol production is expected to reduce the cost of capital and operations and therefore, makes butanol production an economically efficient process10,47,48. An integrated system of simultaneous saccharification, fermentation and product recovery for butanol production from corn stover by Clostridium beijerinckii P260 was reported to be an efficient high butanol productivity of 0.19 g/L.h, while the butanol productivity without product recovery was 0.12 g/L.h47. An efficient process integration for butanol production from whey permeate and recovery by gas stripping using immobilized cells of C. acetobutylicum P262 was reported by Qureshi and Maddox49. This integrated system was operated in continuous fermentation for 4 months in steady rate operation and high productivity of butanol. In situ two-stage gas stripping recovery process was integrated in ABE fermentation for butanol production by Clostridium acetobutylicum JB200 using a fibrous bed bioreactor was found to be highly productive for butanol production compared to fermentation without in situ gas stripping10. The reported integrated process was also effective in production of high butanol concentration and thus the process was energy saving and more economic48.
This study was carried out in a single stage continuous fermentation with free cells of C. acetobutylicum YM1, but for higher butanol productivities, the use of a two-stage continuous fermentation or continuous fermentation using immobilized cultures or biomass-retention cultures are the best choices that have been proposed for higher butanol productivities due to the possibility of sustaining higher dilution rates50,51.
Economic Study
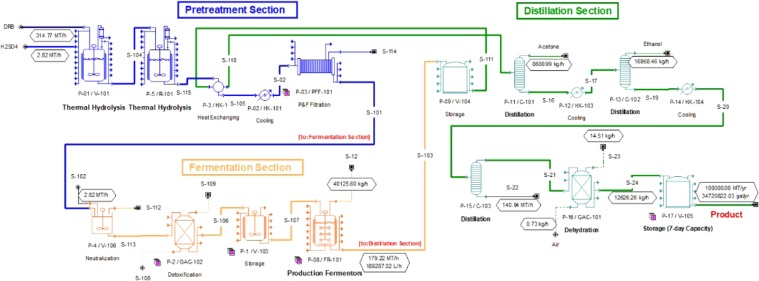
Figure 6 shows the process flow diagram of the plant generated using SuperPro Designer. The process starts with pretreating the DRB with dilute sulfuric acid in two thermal reactors in series. The pretreated DRB will be filtered by passing through a filter press before neutralization. Detoxification column filled with activated carbon will be installed prior to fermentation to remove any inhibitors formed during dilute acid pretreatment. The product will proceed to fermentation process. The reaction will be conducted in a fermenter with seed feeding of C. acetobutylicum YM1. Four major components will be obtained from the fermenter, i.e. butanol, acetone, ethanol and water. Butanol is the desired product with acetone and ethanol as by-products. They will be separated using three distillation columns in view of their different boiling points. In the first distillation column, acetone will be collected as top product, the bottom product will pass through second distillation column and ethanol will be separated as top product. Finally, butanol and water will pass through the third distillation column and butanol will be collected as top product. Dehydration of butanol will be performed to concentrate the product.
Figure 6.
Process flow diagram for continuous butanol fermentation of DRB.
Economy analysis was performed for this plant setup and considering the annual butanol production capacity of 100 × 106 kg from DRB with 330 operating days (equivalent to 7920 h/year). Table 4 shows the fixed capital estimate for production of butanol from DRB. For this plant, total equipment cost was estimated to be $32,385,000. Other significant costs such as installation, process piping, instrumentation, insulation, electrical, buildings, yard improvement and auxiliary facilities were listed in Table 4. The total plant direct cost (TPDC) was projected to be $105,652,000. The total plant indirect cost (TPIC) which includes engineering and construction was $63,391,000 in total, whereby engineering and construction contributed $26,413,000 and $36,978,000, respectively. The total plant cost (TPC) which is the summation of TPDC and TPIC was $169,043,000. Contractor’s fee and contingency (CFC) was $25,357,000 (Contractor’s fee = $8,452,000; Contingency = $16,904,000). These make up the direct fixed capital cost (DFC) of $194,400,000.
Table 4.
Fixed capital estimate for production of butanol from de-oiled rice bran.
| A. Total plant direct cost (TPDC; physical cost) | ($) |
| Equipment purchase cost | 32,385,000 |
| Installation | 12,383,000 |
| Process piping | 11,335,000 |
| Instrumentation | 12,945,000 |
| Insulation | 972,000 |
| Electrical | 3,239,000 |
| Buildings | 14,573,000 |
| Yard improvement | 4,858,000 |
| Auxiliary facilities | 12,954,000 |
| TPDC | 105,652,000 |
| B. Total plant indirect cost (TPIC) | |
| Engineering | 26,413,000 |
| Construction | 36,978,000 |
| TPIC | 63,391,000 |
| C. Total plant cost (TPC = TPDC + TPIC) | 169,043,000 |
| D. Contractor’s fee and contingency (CFC) | |
| Contractor’s fee | 8,452,000 |
| Contingency | 16,904,000 |
| CFC | 25,357,000 |
| E. Direct fixed capital cost (DFC = TPC + CFC) | 194,400,000 |
Table 5 shows the annual operating cost including raw materials, facility and utilities costs. Two different prices of DRB were chosen to estimate the annual operating cost, i.e. DRB at unit price of $50/MT (case 1) and $20/MT (case 2). Approximately 1,246,498 MT of DBR will be used annually. With the basis of DRB unit cost $50 and $20 per MT, annual cost is estimated to be $62,324,922 (for case 1) and $24,929,969 (for case 2), respectively. Apart from DRB, sulfuric acid and sodium hydroxide are the materials used in the plant. This contributed to a total raw material cost of $65,849,436 (case 1) and $28,454,483 (case 2) per year. Cost of facilities and utilities also considered in annual operating cost, as presented in Table 5. The plant projected to consume 27,727,819 kW-h electricity in a year. A unit cost of electivity is $0.01 per kW-h, making an annual electricity cost of $2,772,782. Other utilities are steam, cooling water and chilled water with usage of 1,813,483 MT, 179,715,372 MT and 8,465,034 MT, respectively. With unit price of $12.00, $0.05 and $0.01 per MT, total cost of steam, cooling water and chilled water were $21,761,793, $8,985,769 and $3,386,014, respectively. This make up a total utilities cost of $36,906,357.
Table 5.
Annual operating cost for butanol production from de-oiled rice bran.
| Unit cost ($) | Annual amount | Cost ($/year) | |
|---|---|---|---|
| A. Raw materials ($/MT) | |||
| De-oiled rice bran (case 1) | 50.000 | 1,246,498 | 62,324,922 |
| De-oiled rice bran (case 2) | 20.000 | 1,246,498 | 24,929,969 |
| Sulfuric acid | 70.000 | 22,347.34 | 1,564,314 |
| Sodium hydroxide | 100.000 | 19,602 | 1,960,200 |
| Total (case 1) | 65,849,436 | ||
| Total (case 2) | 28,454,483 | ||
| B. Facility ($/kg MP)* | 366.108 | 36,610,776 | |
| C. Utilities | |||
| Standard power ($/kW-h) | 0.010 | 27,727,81 | 2,772,782 |
| Steam ($/MT) | 12.00 | 1,813,483 | 21,761,793 |
| Cooling water ($/MT) | 0.05 | 179,715,372 | 8,985,769 |
| Chilled water ($/MT) | 0.01 | 8,465,034 | 3,386,014 |
| Total | 36,906,357 | ||
*MP - Total flow of main product (butanol).
Having both fixed capital cost and annual operating cost estimated, the total investment cost to operate this plant is projected to be $213,567,000 for case 1 and $210,168,000 for case 2. Table 6 shows the profitability analysis of the plant. The annual production of butanol, acetone and ethanol produced is projected to be 100,000,000 kg, 68,753,445 kg and 134,390,206 kg. With the basis of $1.48 (butanol), $0.959 (acetone) and $0.90 (ethanol) unit price, it is estimated to generate annual revenue of $334,885,739. With these analyses, the unit production cost of butanol is $1.405/kg for case 1 and $1.031/kg for case 2.
Table 6.
The profitability analysis of production of butanol from de-oiled rice bran in continuous fermentation process.
| Revenue | Amount produced (kg/yr) | Unit selling cost ($/kg) | Annual revenue ($/yr) |
|---|---|---|---|
| Butanol | 100,000,000 | 1.48 | 148,000,000 |
| Acetone | 68,753,445 | 0.959 | 65,934,554 |
| Ethanol | 134,390,226 | 0.90 | 120,951,186 |
Conclusion
Continuous fermentation for butanol production SADRB was successfully performed. Further detoxification of SADRB by activated charcoal significantly reduced fermentation inhibitory compounds and improved the continuous fermentation performance. Pretreatment of de-oiled rice bran followed by detoxification was a necessary process for the enhancement of butanol production. The highest ABE (12.42 g/L), butanol (6.87 g/L) and ABE productivity (0.428 g/L.h) were obtained when detoxified SADRB was utilized in continuous fermentation at a dilution rate of 0.02 h−1 using C. acetobutylicum YM1. The results indicate that continuous fermentation using DRB hydrolysate is a potential as an inexpensive substrate for the high productivity of butanol production. This study presented economic analysis of continuous conversion process of DRB to butanol by C. acetobutylicum YM1. The process is including dilute acid pretreatment of DRB, detoxification of sugars released from the pretreatment, fermentation and recovery of butanol, acetone and ethanol by distillation process. Based on the data from this study, the cost of butanol production estimated as $1.405/kg based on DRB price of $50/MT while in case the DRB price drop to $20/MT, this would reduce the cost of butanol production to $1.031/kg.
Methods
Microorganism
In this study, a local aerotolerant strain of Clostridium acetobutylicum YM1 was used. The inoculum was prepared by activating a spore suspension (1 mL) in 10 mL of a tryptone-yeast extract-acetate medium (TYA) with a subsequent heat shock for 1 min in boiling water, cooling in ice water and then incubation for 1–2 days at 30 °C under anaerobic condition. Before inoculation, the TYA medium was sparged with nitrogen gas (95%) to facilitate anaerobic conditions.
The inoculum was prepared using TYA medium that consisted of 20 g/L glucose, 6 g/L tryptone, 3 g/L ammonium acetate, 2 g/L yeast extract, 0.5 g/L KH2PO4, 0.3 g/L MgSO4.7H2O, and 0.01 g/L FeSO4.7H2O.
Pretreatment of de-oiled rice bran (DRB)
Rice bran was obtained from the Abidin Rice Mill Sdn. Bhd., Perlis, Malaysia, and kept at 4 °C until use. Rice bran was de-oiled by extracting the oil from rice bran using hexane (J.T. Baker Chemical Co. Phillipsburg, NJ, USA), as reported by Al-Shorgani et al.13. The pretreatment with sulfuric acid was carried out by soaking 12% (w/v) of DRB in a 1% (v/v) sulfuric acid solution and then autoclaving it (at 121 °C/15 psi) for 1 h. The solid materials after pretreatment were separated by filtration and the pH of the pretreated DRB with sulfuric acid (SADRB) was adjusted to 6.2 by using 10 M NaOH.
Detoxification of SADRB hydrolysate
Detoxification of the SADRB hydrolysate was applied in order to reduce the concentration of inhibitory compounds such as furfural, HMF, acetic acid, formic acid, and levulinic acid. The SADRB hydrolysate (pH 6) was passed through activated charcoal that was packed in a glass column (60 cm × 2 cm). Ten grams of activated charcoal was used to detoxify 1 L of SADRB hydrolysate. The pH of the detoxified SADRB was adjusted again to a pH of 6.2 before sterilization.
Fermentation
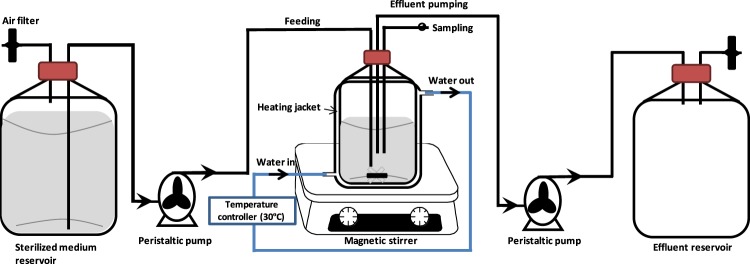
Batch fermentation experiments were conducted in 100-mL serum bottles outfitted with rubber stoppers and crimped with aluminium seals, with a working volume of 80 mL under anaerobic condition. Continuous fermentation was conducted in a 1 L bioreactor (jacketed-Scott Duran bottle) with a working volume of 600 mL. The jacketed-bioreactor vessel was heated by cycling water continuously in the jacket at 30 °C. The medium was pumped at various dilution rates using a peristaltic pump (Masterflex, HV-77120-42, Cole-Parmer Instrument Co., Vernon Hills, IL, USA). Figure 7 shows the schematic diagram of the bioreactor and continuous fermentation system.
Figure 7.
Schematic diagram of the continuous butanol fermentation.
Sterilized SADRB was used as a fermentation medium and stored in a feeding tank that was connected to the fermentation bioreactor. The bioreactor was inoculated using a 10% (v/v) fresh inoculum of C. acetobutylicum YM1 (grown for 20 h) and incubated at 30 °C. The bioreactor was heated at a constant temperature (30 °C) during the continuous fermentation by cycling water in the jacket of the bioreactor. Continuous fermentation was started by feeding SADRB medium at certain flow rates after 24 h of fermentation, in which the bacteria reached stationary phase and entered the solventogenic phase. The volume of the fermentation medium in the bioreactor was kept constant by using the peristaltic pump.
Continuous fermentation was initiated after 24 h of batch fermentation to allow significant bacterial biomass concentration and butanol production. After that, fresh SADRB medium was fed into the fermentor, and the volume of the fermentation medium in the fermentor was maintained at a constant by setting a purge flow with the same volumetric stream rate as the feed flow rate. No nitrogen gas was fed into the culture during the continuous fermentation, and the pH was not controlled during the continuous fermentation. Samples were collected periodically for fermentation monitoring and analysis.
The SADRB hydrolysate was supplemented with TYA ingredients (without glucose) and the pH of the medium was adjusted to 6.2 before sterilization. The fermentation medium was mixed and stirred during the continuous fermentation using a magnetic stirrer at 150 rpm.
Analysis methods
Fermentation samples were collected and centrifuged at 5000 g for 5 min, and the supernatant was used for the analysis of solvents, acids and sugars.
The analysis of acetone, butanol, ethanol, acetic acid and butyric acid was performed using a gas chromatograph (7890A GC-System, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and a 30-m capillary column (Equity-1; 30 m × 0.32 mm × 1.0 µm film thickness; Supelco Co., Bellefonate, PA, USA). The injection temperature was set at 250 °C and the detection temperature was set at 280 °C. The carrier gas used was helium at a flow rate of 1.5 mL/min.
Inhibitory compounds such as furfural, HMF, acetic acid, formic acid, and levulinic acid were measured using a high-performance liquid chromatograph (HPLC; 12000 Series, Agilent Technologies, Palo Alto, CA, USA). Separations and concentrations were performed on a Phenomenex C18 column (250 × 4.6 mm ID; Phenomenex Inc., Torrance, CA, USA) using a UV detector at 220 nm (UV-D; 1200, Agilent Technologies, Palo Alto, CA, USA) at 40 °C. The mobile phase was a mixture of 95% sulfuric acid (20 mM) and 5% acetonitrile, with an overall flow rate of 1 mL/min.
Sugars including glucose, xylose and cellobiose were estimated by HPLC (12000 Series, Agilent Technologies, Palo Alto, CA, USA) using a Shodex Asahipak NH2P-50 4E column (4.6 mm ID × 250 mm; Shodex, Kanagawa, Japan). Sugar concentrations were measured with a refractive index detector (RID; 1200, Agilent Technologies, Palo Alto, CA, USA) at 30 °C with a mobile phase flow rate of 1 mL/min, with a mixture of acetonitrile (60%) and water (40%).
The concentrations of total reducing sugars were measured using the 3,5-dinitrosalicylic acid (DNS) assay according to Miller’s method52. The bacterial biomass concentration was estimated as dry cell weight (DCW).
The volumetric productivity for ABE or butanol in batch fermentation was calculated according to the Equation 1 while the volumetric productivity of ABE or butanol in continuous fermentation was calculated by Equation 2. Equation 3 was used to define the yield of ABE and butanol.
| 1 |
| 2 |
| 3 |
Financial Evaluation
The production of butanol was simulated in SuperPro Designer (version 8.5003, Intelligen Inc.) for basis of 330 days/year. In the simulation, material and energy balance was computed with the basis of annual butanol production of 100,000 MT. The economic assessment was conducted in SuperPro economic evaluation where the butanol is produced continuously in a fermenter as main product and acetone and ethanol are by-products. The price value and calculation were based on year 2018. In this analysis, site development, transportation and mechanical pretreatment of DRB were not included.
Supplementary information
Author List Changes Approval form_SREP-18-02725C
Acknowledgements
This study was financially supported by Universiti Kebangsaan Malaysia through grants; GUP-2016-006 and DIP-2017-019. Authors would like to thank Dr. Hafiza Shukor from Universiti Malaysia Perlis for assistance.
Author Contributions
Najeeb Kaid Nasser Al-Shorgani designed and performed the experiments, interpreted the data and drafted the manuscript, Mohd Sahaid Kalil conceived of the study, participated in its design and helped to draft the manuscript, Abdualati Ibrahim Al-Tabib & Abudukeremu Kadier performed some experimental procedures, Mohd Fauzi Zanil and Kiat Moon Lee performed the economic analysis.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40840-y.
References
- 1.Zhao X, Condruz S, Chen J, Jolicoeur M. A quantitative metabolomics study of high sodium response in Clostridium acetobutylicum ATCC 824 acetone-butanol-ethanol (ABE) fermentation. Sci Rep. 2016;6:28307. doi: 10.1038/srep28307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shorgani NKN, Isa MHM, Yusoff WMW, Kalil MS, Hamid AA. Isolation of a Clostridium acetobutylicum strain and characterization of its fermentation performance on agricultural wastes. Renewable Energy. 2016;86:459–465. doi: 10.1016/j.renene.2015.08.051. [DOI] [Google Scholar]
- 3.Azman NF, et al. Biohydrogen production from de-oiled rice bran as sustainable feedstock in fermentative process. International Journal of Hydrogen Energy. 2016;41:145–156. doi: 10.1016/j.ijhydene.2015.10.018. [DOI] [Google Scholar]
- 4.Qureshi N, Li XL, Hughes S, Saha BC, Cotta MA. Butanol production from corn fiber xylan using Clostridium acetobutylicum. Biotechnology Progress. 2006;22:673–680. doi: 10.1021/bp050360w. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi N, Blaschek HP. Butanol production using Clostridium beijerinckii BA101 hyper-butanol producing mutant strain and recovery by pervaporation. Applied Biochemistry and Biotechnology. 2000;84-86:225–235. doi: 10.1385/ABAB:84-86:1-9:225. [DOI] [PubMed] [Google Scholar]
- 6.Xue C, Zhao J, Chen L, Yang S-T, Bai F. Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum. Biotechnology Advances. 2017;35:310–322. doi: 10.1016/j.biotechadv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Maddox IS. The acetone-butanol-ethanol fermentation: Recent progress in technology. Biotechnology and Genetic Engineering Reviews. 1989;7:189–220. doi: 10.1080/02648725.1989.10647859. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi N, Schripsema J, Lienhardt J, Blaschek HP. Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World Journal of Microbiology and Biotechnology. 2000;16:377–382. doi: 10.1023/a:1008984509404. [DOI] [Google Scholar]
- 9.Xue C, Zhao X-Q, Liu C-G, Chen L-J, Bai F-W. Prospective and development of butanol as an advanced biofuel. Biotechnology Advances. 2013;31:1575–1584. doi: 10.1016/j.biotechadv.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Xue C, et al. Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresource Technology. 2013;135:396–402. doi: 10.1016/j.biortech.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 11.Chen CK, Blaschek HP. Acetate enhances solvent production and prevents degeneration in Clostridium beijerinckii BA101. Appl Microbiol Biotechnol. 1999;52:170–173. doi: 10.1007/s002530051504. [DOI] [PubMed] [Google Scholar]
- 12.Childs, N. & Skorbiansky, S. R. Rice outlook, https://www.ers.usda.gov/webdocs/publications/84341/rcs-17g.pdf?v=42930 (2017).
- 13.Al-Shorgani NKN, Kalil MS, Yusoff WMW. Biobutanol production from rice bran and de-oiled rice bran by Clostridium saccharoperbutylacetonicum N1-4. Bioprocess Biosyst Eng. 2012;35:817–826. doi: 10.1007/s00449-011-0664-2. [DOI] [PubMed] [Google Scholar]
- 14.Ezeji T, Qureshi N, Blaschek HP. Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnology and Bioengineering. 2007;97:1460–1469. doi: 10.1002/bit.21373. [DOI] [PubMed] [Google Scholar]
- 15.Kumar AK, Sharma S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresources and Bioprocessing. 2017;4:7. doi: 10.1186/s40643-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jönsson LJ, Martín C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresource Technology. 2016;199:103–112. doi: 10.1016/j.biortech.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi N, et al. Butanol production by Clostridium beijerinckii. Part I: use of acid and enzyme hydrolyzed corn fiber. Bioresource Technology. 2008;99:5915–5922. doi: 10.1016/j.biortech.2007.09.087. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Ying Y, Li F, Ma C, Xu P. Butanol production by Clostridium beijerinckii ATCC 55025 from wheat bran. Journal of Industrial Microbiology & Biotechnology. 2010;37:495–501. doi: 10.1007/s10295-010-0695-8. [DOI] [PubMed] [Google Scholar]
- 19.Jones D, Woods D. Acetone-butanol fermentation revisited. Microbiological Reviews. 1986;50:484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu P-J, Lin H-C, Yu W-T, Chern J-M. Chemical regeneration of activated carbon used for dye adsorption. Journal of the Taiwan Institute of Chemical Engineers. 2011;42:305–311. doi: 10.1016/j.jtice.2010.06.001. [DOI] [Google Scholar]
- 21.Sabio E, et al. Thermal regeneration of activated carbon saturated with p-nitrophenol. Carbon. 2004;42:2285–2293. doi: 10.1016/j.carbon.2004.05.007. [DOI] [Google Scholar]
- 22.Li Y, et al. Study on regeneration of waste powder activated carbon through pyrolysis and its adsorption capacity of phosphorus. Scientific Reports. 2018;8:778. doi: 10.1038/s41598-017-19131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narbaitz RM, Karimi-Jashni A. Electrochemical regeneration of granular activated carbons loaded with phenol and natural organic matter. Environmental Technology. 2009;30:27–36. doi: 10.1080/09593330802422803. [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Sorial GA. Chemical activation of bituminous coal for hampering oligomerization of organic contaminants. Journal of Hazardous Materials. 2011;197:311–319. doi: 10.1016/j.jhazmat.2011.09.093. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Tu M, Xie R, Adhikari S, Tong Z. A comparison of three pH control methods for revealing effects of undissociated butyric acid on specific butanol production rate in batch fermentation of Clostridium acetobutylicum. AMB Express. 2013;3:3–3. doi: 10.1186/2191-0855-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho DH, Shin S-J, Kim YH. Effects of acetic and formic acid on ABE production by Clostridium acetobutylicum and Clostridium beijerinckii. Biotechnol Bioproc E. 2012;17:270–275. doi: 10.1007/s12257-011-0498-4. [DOI] [Google Scholar]
- 27.Monot F, Engasser J-M, Petitdemange H. Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch cultures of Clostridium acetobutylicum. Applied Microbiology and Biotechnology. 1984;19:422–426. doi: 10.1007/bf00454381. [DOI] [Google Scholar]
- 28.Wang S, et al. Formic acid triggers the “Acid Crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Appl Environ Microbiol. 2011;77:1674–1680. doi: 10.1128/aem.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudahettige-Nilsson RL, et al. Biobutanol production by Clostridium acetobutylicum using xylose recovered from birch Kraft black liquor. Bioresource Technology. 2015;176:71–79. doi: 10.1016/j.biortech.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Mussatto SI, Roberto IC. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresource Technology. 2004;93:1–10. doi: 10.1016/j.biortech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Kamal SMM, Mohamad NL, Abdullah AGL, Abdullah N. Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Procedia Food Science. 2011;1:908–913. doi: 10.1016/j.profoo.2011.09.137. [DOI] [Google Scholar]
- 32.Guo X, Cavka A, Jönsson LJ, Hong F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microbial Cell Factories. 2013;12:93. doi: 10.1186/1475-2859-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew ST, Arbakariya A, Rosfarizan M, Raha AR. Production of solvent (acetone-butanol-ethanol) in continuous fermentation by Clostridium saccharobutylicum DSM 13864 using gelatinised sago starch as a carbon source. Malaysian Journal of Microbiology. 2006;2:42–50. [Google Scholar]
- 34.Ni Y, Xia Z, Wang Y, Sun Z. Continuous butanol fermentation from inexpensive sugar-based feedstocks by Clostridium saccharobutylicum DSM 13864. Bioresource Technology. 2013;129:680–685. doi: 10.1016/j.biortech.2012.11.142. [DOI] [PubMed] [Google Scholar]
- 35.Godin C, Engasser JM. Improved stability of the continuous production of acetone-butanol by Clostridium acetobutylicum in a two-stage process. Biotechnol Lett. 1988;10:389–392. doi: 10.1007/bf01087434. [DOI] [Google Scholar]
- 36.Mollah AH, Stuckey DC. The influence of H2, CO2 and dilution rate on the continuous fermentation of acetone-butanol. Appl Microbiol Biotechnol. 1992;37:533–538. doi: 10.1007/bf00240720. [DOI] [Google Scholar]
- 37.Andersch W, Bahl H, Gottschalk G. Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1983;18:327–332. doi: 10.1007/bf00504740. [DOI] [Google Scholar]
- 38.Al-Shorgani NKN, Al-Tabib AI, Kalil MS. Production of butanol from acetyl chloride-treated deoiled rice bran by Clostridium acetobutylicum YM1. BioResources. 2017;12:8505–8518. [Google Scholar]
- 39.Liu K, et al. Butanol production from hydrothermolysis-pretreated switchgrass: Quantification of inhibitors and detoxification of hydrolyzate. Bioresour Technol. 2015;189:292–301. doi: 10.1016/j.biortech.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Villarreal MLM, Prata AMR, Felipe MGA, Almeida E, Silva JB. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzyme and Microbial Technology. 2006;40:17–24. doi: 10.1016/j.enzmictec.2005.10.032. [DOI] [Google Scholar]
- 41.Wang L, Chen H. Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochemistry. 2011;46:604–607. doi: 10.1016/j.procbio.2010.09.027. [DOI] [Google Scholar]
- 42.Dolejs I, Krasnan V, Stloukal R, Rosenberg M, Rebros M. Butanol production by immobilised Clostridium acetobutylicum in repeated batch, fed-batch, and continuous modes of fermentation. Bioresour Technol. 2014;169:723–730. doi: 10.1016/j.biortech.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 43.Ezeji TC, Qureshi N, Blaschek HP. Continuous butanol fermentation and feed starch retrogradation: butanol fermentation sustainability using Clostridium beijerinckii BA101. J Biotechnol. 2005;115:179–187. doi: 10.1016/j.jbiotec.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, et al. Continuous butanol fermentation from xylose with high cell density by cell recycling system. Bioresource Technology. 2013;129:360–365. doi: 10.1016/j.biortech.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 45.Chen C, et al. Acetone–butanol–ethanol fermentation in a continuous and closed-circulating fermentation system with PDMS membrane bioreactor. Bioresource Technology. 2013;128:246–251. doi: 10.1016/j.biortech.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 46.Xue C, et al. A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production. Biotechnology and Bioengineering. 2016;113:120–129. doi: 10.1002/bit.25666. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi N, et al. Process integration for simultaneous saccharification, fermentation, and recovery (SSFR): Production of butanol from corn stover using Clostridium beijerinckii P260. Bioresource Technology. 2014;154:222–228. doi: 10.1016/j.biortech.2013.11.080. [DOI] [PubMed] [Google Scholar]
- 48.Xue C, et al. Integrated butanol recovery for an advanced biofuel: current state and prospects. Appl Microbiol Biotechnol. 2014;98:3463–3474. doi: 10.1007/s00253-014-5561-6. [DOI] [PubMed] [Google Scholar]
- 49.Qureshi N, Maddox IS. Integration of continuous production and recovery of solvents from whey permeate: use of immobilized cells of Clostridium acetobutylicum in a flutilized bed reactor coupled with gas stripping. Bioprocess Engineering. 1990;6:63–69. doi: 10.1007/bf00369279. [DOI] [Google Scholar]
- 50.Mutschlechner O, Swoboda H, Gapes JR. Continuous two-stage ABE-fermentation using Clostridium beijerinckii NRRL B592 operating with a growth rate in the first stage vessel close to its maximal value. J. Mol. Microbiol. Biotechnol. 2000;2:101–105. [PubMed] [Google Scholar]
- 51.Gallazzi A, Branska B, Marinelli F, Patakova P. Continuous production of n-butanol by Clostridium pasteurianum DSM 525 using suspended and surface-immobilized cells. Journal of Biotechnology. 2015;216:29–35. doi: 10.1016/j.jbiotec.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 53.Badr HR, Toledo R, Hamdy MK. Continuous acetone-ethanol-butanol fermentation by immobilized cells of Clostridium acetobutylicum. Biomass and Bioenergy. 2001;20:119–132. doi: 10.1016/S0961-9534(00)00068-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author List Changes Approval form_SREP-18-02725C