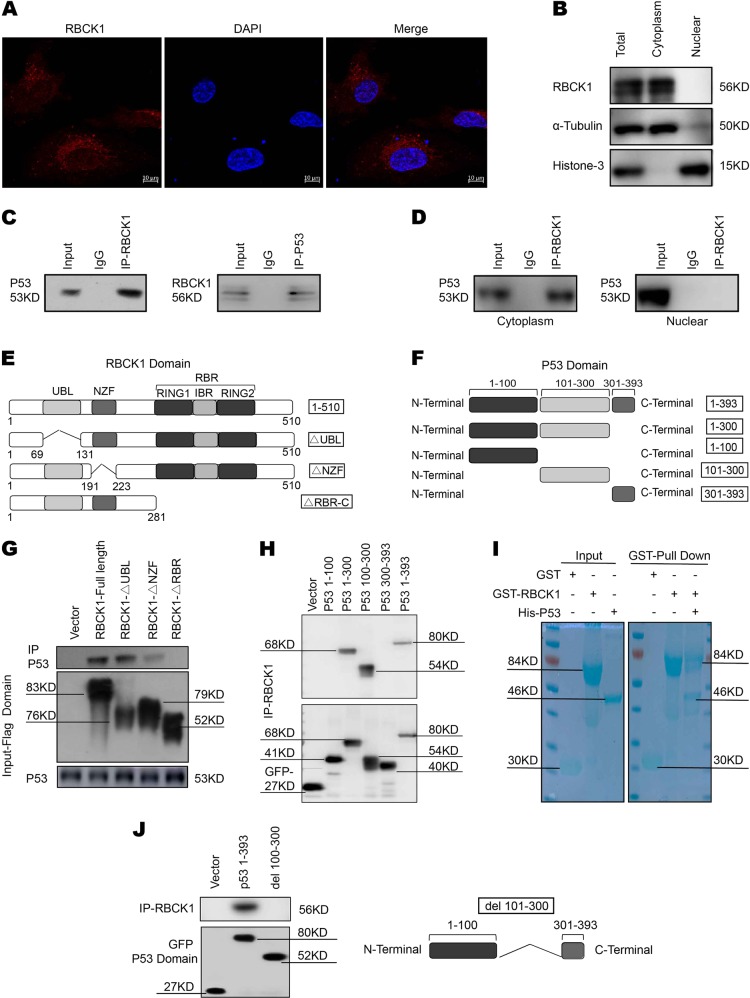
Fig. 7. Intracellular localization of RBCK1 and its interaction with p53.
a Intracellular localization analysis of RBCK1 by immunofluorescence assay. Caki-1 cells were cultured in phenol red-free DMEM medium. Intracellular localization of RBCK1 (red) were shown. Nuclei (blue) were stained with 4′,6-diamidino-2-phenylindole (DAPI). b RBCK1 is mainly localized in the cytoplasm. The subcellular protein fractionation kit (Thermo scientific, 78840) was used for cytoplasm and nuclear separation. Tubulin and Histone-3 were used for cytoplasm and nuclear control. c Co-IP assays reveal associations between endogenous RBCK1 and p53 in Caki-1 cells. IgG was used as a control. Caki-1 cells were harvested with NP-40 lysis buffer. CO-IP was performed using antibody as indicated. d Nuclear and cytoplasmic separation-based Co-IP. The subcellular protein fractionation kit was used for cytoplasm and nuclear separation. Based on the separation, IP was done by RBCK1 antibody in both the cytosol and nuclear lysis. p53 antibody was used to detect the interaction in both the cytosol and nuclear. e RBCK1 domain structure and deletion mutants used in the study (Full length, ΔUBL, ΔNZF, ΔRBR-C). f P53 domain structure and deletion mutants used in the study (Full length, aa1–300, aa1–100, aa101–300, aa301–393). g HEK293 cells were transfected with 2 μg EGFP-p53 together with Flag-RBCK1 full length or mutants (ΔUBL, ΔNZF, ΔRBR-C). After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using EGFP antibody. The possible interacted RBCK1 domains were detected by flag antibody. h HEK293 cells were transfected with 2 μg Flag-RBCK1 together with EGFP-p53 full length or mutants (aa1–300, aa1–100, aa101–300, aa301–393). After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using flag antibody. The possible interacted p53 domains were detected by EGFP antibody. i Pulldown assay experiment. p53 fragment was individually expressed as His-fusion protein. GST-fusion RBCK1 protein was purified using glutathione-Sepharose beads according to the Manufacturer’s protocol. j Co-IP shows that mutants p53 del 100–300 fails to interact with RBCK1. HEK293 cells were transfected with 2 μg Flag-RBCK1 together with EGFP-p53 full length or del 100–300 mutants. After 24 h, cells were harvested with NP-40 lysis buffer. Co-IP was performed using flag antibody