Figure 6. Summary of SHIV detection and proportion of animals with detectable cell-free or cell-associated SHIV in breast milk mAb-infused, orally SHIV-challenged infant RMs.
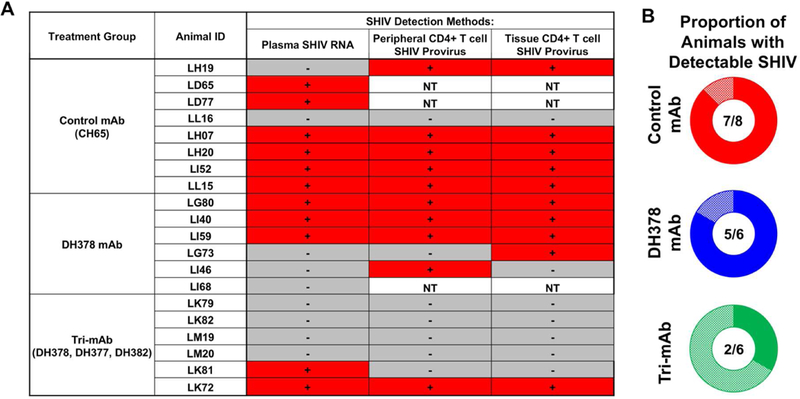
A) Binary indication of detectable plasma SHIV RNA, peripheral CD4+ T cell SHIV provirus, and tissue CD4+ T cell SHIV provirus detection. For each animal, SHIV detection methods with detectable SHIV in any tested tissue or at any timepoint were indicated with a red “+”, while those with undetectable values in all tested tissues and at all timepoints were indicated with a grey “-”. B) Proportion of animals with detectable cell-free or cell-associated SHIV as defined by detectable SHIV plasma RNA, SHIV provirus in tissues or PBMCs, tissue mononuclear cell-associated infectious SHIV titers, or SHIV RNA-transcribing cells.
