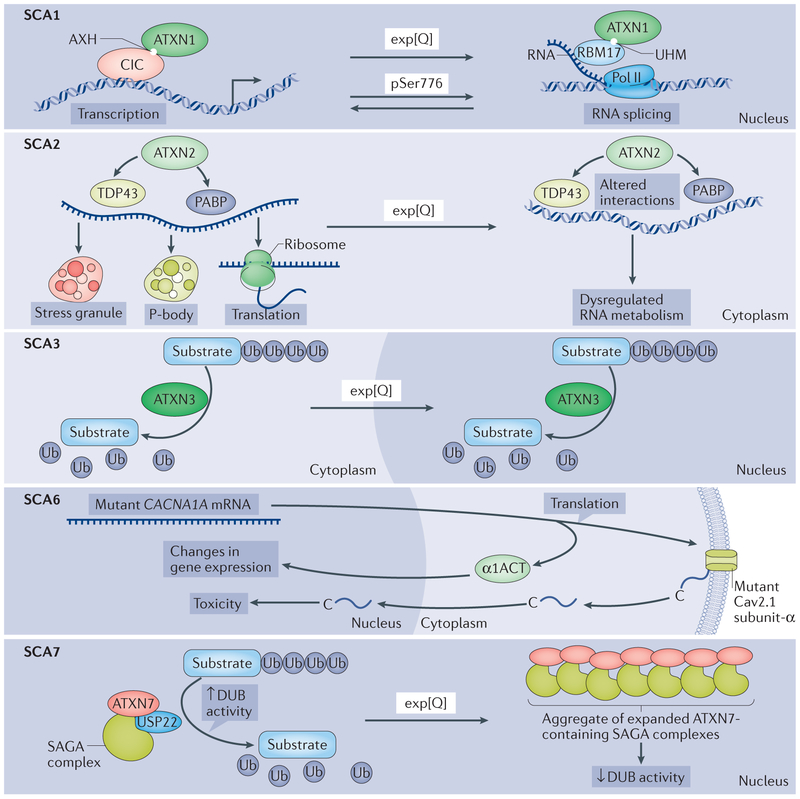
Figure 2 |. Cellular processes affected by mutant polyglutamine proteins in spinocerebellar ataxias.
Each panel illustrates the cellular processes affected by the polyglutamine (polyQ) proteins in spinocerebellar ataxia 1 (SCA1), SCA2, SCA3, SCA6 and SCA7. In all of the SCAs depicted, except SCA3, the cerebellar Purkinje cells are prominently affected, whereas in SCA3, the Purkinje cells are more mildly affected. Top panel (SCA1): in the ataxin 1 (ATXN1) protein, it is believed that the AXTN1 HBP1 (AXH) domain and the U2AF homology (UHM) domain are interaction motifs for the transcriptional regulator capicua (CIC) and the RNA splicing factor RBM17, respectively, and a shift in these interactions is critical for mutant, polyglutamine-expanded ATXN1 to cause disease in Purkinje cells. PolyQ expansion is depicted by ‘exp[Q]’ in all panels. Phosphorylation of Ser776 in ATXN1 (pSer 776) also shifts the balance between these interactions. Second panel (SCA2): ATXN2 is known to interact directly or indirectly with numerous proteins implicated in RNA metabolism, as well as RNA itself, to regulate translation, stress granule formation and P-body formation. Of particular interest are the interactions of ATXN2 with polyadenylate-binding protein (PABP) and TAR DNA-binding protein 43 (TDP43), each of which also binds directly to RNA. One hypothesis is that the polyQ tract length in ATXN2 impairs interactions with PABP and TDP43 and thereby contributes to SCA2 pathogenesis, as well as the risk for ALS (not shown). Third panel (SCA3): as a deubiguitinase (DUB), ATXN3 binds and cleaves polyubiguitin chains and has been implicated in a variety of ubiguitin (Ub)-dependent protein quality control pathways. Although expanded ATXN3 retains DUB activity in vitro, changes in polyQ-repeat length may alter its function in the complex cellular environment, with deleterious consequences. As with many polyQ disease proteins, mutant ATXN3 becomes concentrated in the nucleus. Fourth panel (SCA6): the CACNA1A gene encodes a bicistronic mRNA that, on translation, yields the following two proteins: the membrane-localized α1A subunit of the Cav2.1 channel and the transcription factor α1ACT. Expansion of the polyQ-encoding repeat in CACNA1A leads to toxicity through altered α1ACT-mediated regulation of transcription, as well as through nuclear translocation of a peptide cleaved from the carboxyl terminus of the mutant Cav2.1 channel subunit. Fifth panel(SCA7): ATXN7 is a component of the SPT-ADA-GCN5 acetyltransferase (SAGA) complex. SAGA regulates transcription through its dual histone-modifying enzymes, the histone acetyltransferase GCN5 and the DUB ubiguitin C-terminal hydrolase 22 (USP22). PolyQ-expanded ATXN7 forms insoluble complexes that are thought to sequester other components of the DUB module such that the SAGA complex can no longer remove ubiquitin from its substrates. Pol II, polymerase II.