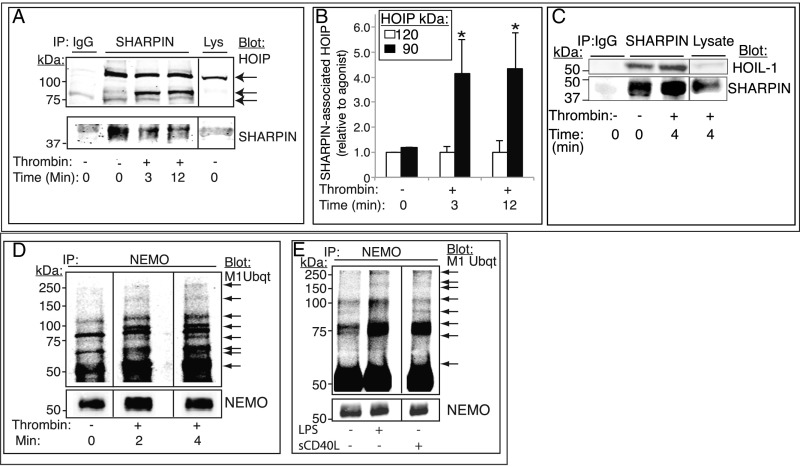
Fig. 3.
LUBAC-mediated Met1 ubiquitination of NEMO and NF-κB pathway signaling are triggered in platelets by hemostatic and inflammatory agonists. (A–C) SHARPIN association with HOIP (A and B) and HOIL-1 (C) was analyzed in SHARPIN immunoprecipitates from human platelets that had been incubated with vehicle or thrombin (1 U/mL). (A) Western blot analysis with an antibody specific for the C-terminal LDD domain of HOIP revealed a full-length 120-kDa band under all incubation conditions and additional 90-kDa cleavage bands in thrombin-stimulated platelets. (B) Extent of HOIP associated with SHARPIN, determined by densitometry and normalized to SHARPIN intensity (n = 4; mean ± SEM; *P < 0.05). (D and E) Met1 linear ubiquitination of NEMO. Platelets were incubated in the absence or presence of 1 U/mL thrombin for 0–4 min (D) or with either 1 μg/mL LPS or 1 μg/mL sCD40L for 4 min (E). Lysates were immunoprecipitated with an anti-NEMO antibody and probed on Western blots for Met1 ubiquitin. Arrows indicate new or more intense Met1 ubiquitin bands of variable molecular sizes following agonist stimulation. All samples within a panel were run on the same gel, and an 8% aliquot of each immunoprecipitation sample was run separately to determine gel loading. The data shown are representative of at least three separate experiments.