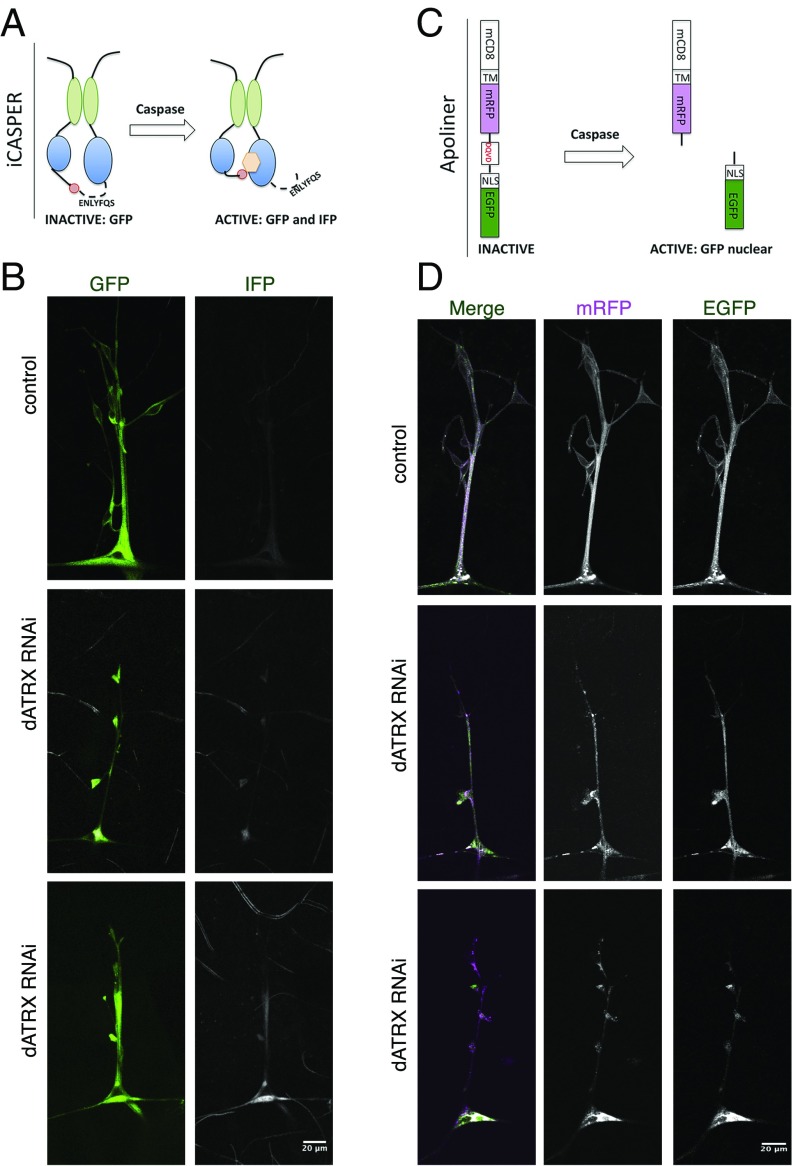
Fig. 6.
Loss of dATRX expression in glia leads to caspase activation. (A) Schematic depicts the iCASPER construct, composed of split-GFP domains as well as a caspase cleavage site that controls the positioning of chromophore for an infrared fluorescent protein. Caspase activity leads to conformation change that permits chromophore incorporation and IFP fluorescence. GFP fluorescence is seen in both the presence and absence of caspase activity. (B) Third-instar larvae expressing RepoGal4 > iCasper or RepoGal4 > iCasper, dATRX RNAi were imaged in the GFP and far red channel to visualize glia membranes (green) and the caspase activity detected by the iCasper sensor (gray). (Scale bar, 20 μm.) (C) Schematic representation of the Apoliner construct. In absence of caspase the GFP and mRFP are linked together through a short peptide and retained at the plasma membrane due to the transmembrane domain attached to mRFP. In the presence of active caspase, the linker is cleaved leading to GFP translocation from the plasma membrane while the mRFP is still attached to the membrane. The lack of colocalization of mRFP and GFP indicates caspase activity. (D) Third-instar larvae expressing RepoGal4 > Apoliner or RepoGal4 > Apoliner, dATRX RNAi were imaged in the GFP and RFP channel to visualize activity of caspase in glia membranes. GFP and mRFP and completely colocalized in control animals; however, in dATRX knockdown larvae, GFP and mRFP are no longer localized indicating caspase activation. (Scale bar, 20 μm.)