Scheme 1. Synthesis of Compounds 6a–6l and 6o–6p.
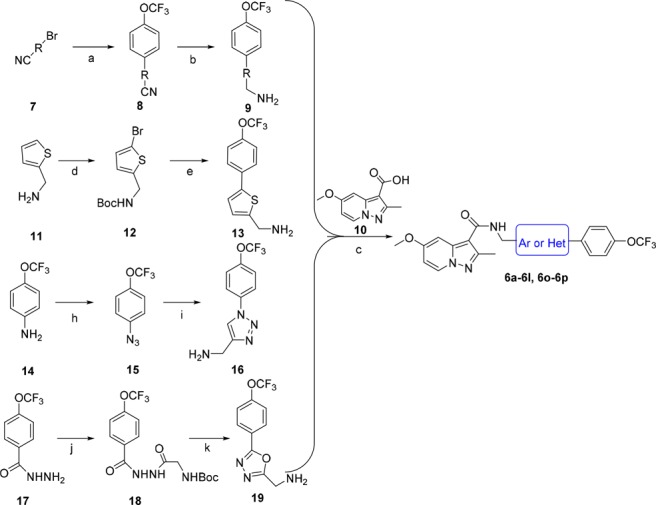
Reagents and conditions: (a) (4-(trifluoromethoxy)phenyl)boronic acid, Pd(PPh3)4, Na2CO3, toluene, 110°C, overnight, 83–92%; (b) LiAlH4, THF, −40°C, 3 h, 48–53%; (c) HATU, DIPEA, DCM, rt, overnight, 49–53%; (d) i. NaHCO3, Boc2O, THF, rt, 3 h, 97%; ii. NBS, DMF, rt, 5 h, 82%; (e) i. 4-(trifluoromethoxy)phenylboronic acid, K2CO3, Pd(PPh3)4, DME, 80°C, 4 h, 87%; ii. TFA, DCM, rt, 5 h, 82%; (h) hydrochloric acid, NaNO2, NaN3, H2O, 0 °C → rt, 2.5 h, 92%; (i) 2-propynylamine, CuI, THF, DIPEA, rt, overnight, 70%; (j) Boc-glycine, HATU, DIPEA, DMF, rt, overnight, 85%; (k) i. Et3N, PPh3, CCl4, DMF, rt, overnight, 68%; ii TFA, DCM, rt, 2 h, 98%.
