Abstract
Menkes disease is a lethal X-linked recessive disorder of copper metabolism caused by mutations in ATP7A, a copper-transporting ATPase with diverse and important biological functions. Partial deficiency of dopamine-beta-hydroxylase is a biochemical hallmark of this illness due to the normal role of ATP7Ain delivery of copper as an enzymatic cofactor. We exploited this fact to develop a diagnostic test for Menkes disease, which proved highly sensitive and specific. The assay has enabled early identification of affected patients, leading to enhanced survival and improved neurodevelopment after early copper treatment, including some completely normal outcomes. In preclinical efforts to develop improved therapies for patients with non-copper-responsive ATP7A mutations, we used brain-directed adeno-associated viral gene therapy to rescue a murine model of the disease. Statistically significant improvement in brain catechol ratios correlated with enhanced survival, and cerebrospinal fluid catechols represent candidate surrogate markers of treatment effect in a future gene therapy clinical trial.
1. INTRODUCTION
The P-type ATPase copper-transporting ATPase 1 (ATP7A) is a major component of the intracellular copper homeostasis apparatus (Kaler, 2011). Defects in ATP7A are responsible for Menkes disease and occipital horn syndrome (OHS) (Chelly et al., 1993; Kaler et al., 1994, Mercer et al., 1993, Vulpe, Levinson, Whitney, Packman, & Gitschier, 1993). The defects in ATP7A encompass a diverse range of genetic mutations, a considerable proportion of which lead to complete loss of function of the transporter (Tümer, 2013). Menkes disease is an often lethal X-linked recessive condition characterized by infantile-onset cerebral and cerebellar neurodegeneration, failure to thrive, coarse hair, and connective tissue abnormalities (Kaler, Westman, et al., 1993). In contrast, the OHS has a much milder and nonlethal neurological phenotype (Das et al., 1995; Kaler et al., 1994; Tang, Robertson, Lem, Godwin, & Kaler, 2006). These conditions occur at an estimated frequency of 1 in 50,000–100,000 newborns. Both conditions are linked to a biochemical phenotype (low copper levels in the blood and a copper deficiency in the brain) that denotes abnormal copper metabolism (Kaler, 1994). Enzymes that depend on copper as an electron-transferring cofactor show decreased activities. Passage of copper across the blood-cerebrospinal fluid (CSF) barrier and the blood-brain barrier (BBB) is likely mediated by ATP7A (Donsante, Johnson, Jansen, & Kaler, 2010; Donsante et al., 2011), and the success of early treatment in cases of Menkes disease seems to rely on residual copper transport activity mediated by mutant ATP7A molecules (Kaler, 1996; Kaler et al., 2008).
Among its multiple cellular tasks, ATP7A transfers copper to dopamine-beta-hydroxylase (DBH) within the lumen of the Golgi network or secre-tory granules (Fig. 11.1) where DBH mediates the conversion of dopamine to norepinephrine. Mutations in ATP7A, or its murine homologue, Atp7a, impair this process, resulting in distinctively abnormal neurochemical patterns, including markedly elevated ratios of non-beta-hydroxylated/beta-hydroxylated metabolites in the catecholamine biosynthetic pathway (Christodoulou et al., 1998; Donsante et al., 2011, 2013; Goldstein, Holmes, & Kaler, 2009; Kaler et al., 2008; Kaler, Goldstein, Holmes, Salerno, & Gahl, 1993). These neurochemical consequences mirror those described in patients with congenital absence of DBH, an autosomal recessive trait (Biaggioni & Robertson, 1987; Robertson et al., 1991; Senard & Rouet, 2006).
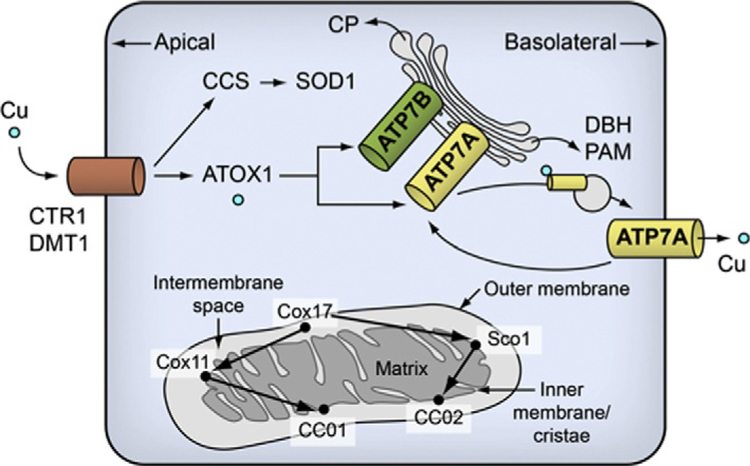
Figure 11.1.
Overview of cellular copper metabolism. In neuronal cells, ATP7A is required for maturation of DBH and PAM. ATOX1, copper transport protein ATOX1; ATP7A, copper-transporting ATPase 1; ATP7B, copper-transporting ATPase 2; CCS, copper chaperone for SOD1; CCO, cytochrome c oxidase; COX11, cytochrome c oxidase assembly protein Cox11; Cox17, cytochrome c oxidase copper chaperone; CP, ceruloplasmin; CTR, copper transporter 1; DBH, dopamine-β-hydroxylase; DMT1, divalent metal transporter 1; PAM, peptidylglycine α-amidating monooxygenase; Sco1, protein SCO1 homologue; SOD1, superoxide dismutase. Reproduced from Kaler (2011), with permission.
In this chapter, we review our extensive experience with the use of plasma catechol ratios for diagnosis of Menkes disease and its variants. We also highlight the more recent use of brain catechol ratios as excellent bio-markers for response to experimental therapeutics in a Menkes disease mouse model.
2. NEONATAL DIAGNOSIS OF MENKES DISEASE BY PLASMA CATECHOL ANALYSIS
In norepinephrinergic neurons, the importance of ATP7A relates to provision of copper to the DBH apoenzyme. DBH is present only in the vesicles of norepinephrinergic neurons where it converts dopamine to the neurotransmitter norepinephrine (Fig. 11.2). We exploited the deficiency of DBH in Menkes disease patients with deficiency or impaired function of ATP7A to prospectively evaluate the diagnostic utility of plasma neurochemical levels (Kaler et al., 2008). We used high-performance liquid chromatography high performance liquid chromatography (HPLC) with electrochemical detection to quantitate the endogenous catechol levels (Holmes, Eisenhofer, & Goldstein, 1994). Before assaying samples, we typically review background information on them. The quality and value of the results depend crucially on how the sample is collected—including factors such as diet, medications, and even posture—and how the sample is handled and stored. Most newborn infants are not receiving medication, although occasionally we have received specimens from critically ill infants on a dopa-mine drip, which obscured the catechol profile.
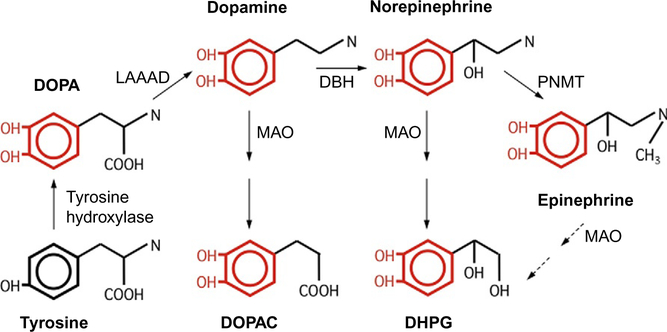
Figure 11.2.
The catecholamine biosynthetic pathway. Tyrosine hydroxylation represents the rate-limiting step.
The steps in our assay are fairly straightforward (Møller et al., 2011) and depend on alumina, which is aluminum oxide. Alumina has the remarkable property of adsorbing catechols in an alkaline environment and desorbing catechols in acidic conditions. Our assay is based on this principle. We first load plasma on the alumina and raise the pH so that the catechols stick to the alumina, wash the alumina, and then lower the pH to elute the alumina-bound catechols. Six catechols are normally present in human plasma: three catecholamines—dopamine, norepinephrine, and epinephrine— and dihydroxyphenylalanine (DOPA), a catecholamine precursor; dihydroxyphenylacetic acid (DOPAC), the deaminated metabolite of dopa-mine; and dihydroxyphenylglycol (DHPG), the deaminated metabolite of norepinephrine and epinephrine (Fig. 11.2). DOPA is the immediate product of the rate-limiting enzymatic step in catecholamine synthesis, hydroxylation of tyrosine, a noncatechol. DOPA is then decarboxylated to produce the catecholamine, dopamine. DBH converts dopamine to norepinephrine. In the adrenal medulla, cells that express the enzyme phenylethanolamine-N-methyltransferase convert norepinephrine to epinephrine. Dopamine is ultimately broken down to DOPAC and norepinephrine to DHPG by monoamine oxidase and other enzymes (Fig. 11.2).
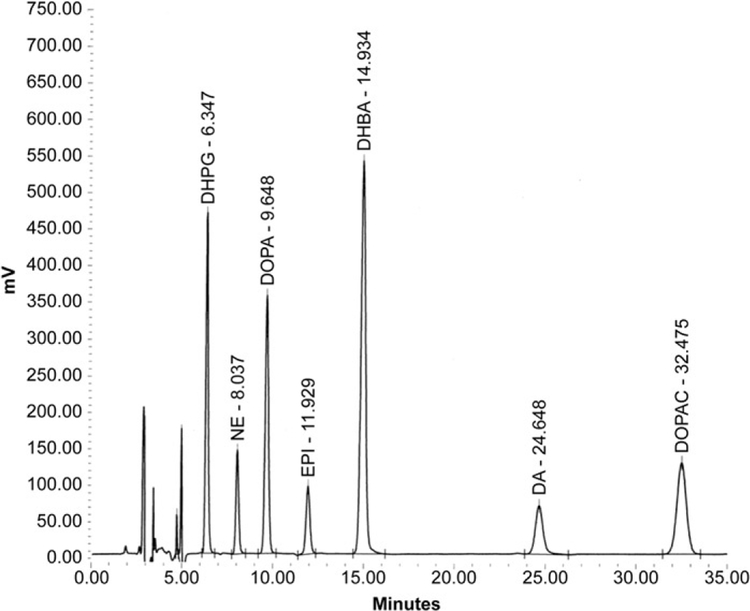
After the catechols have been purified partially by alumina extraction, the supernate is injected into a HPLC-electrochemical detection system, which is commonly employed to separate compounds of interest. HPLC is the means to separate the catechols, and electrochemical detection is the means to quantify the amount of catechols. The numbers above the peak are the retention times of the compound on the HPLC column, that is, how long the compound is retained on the column before elution (Fig. 11.3). For a given set of conditions (column age, temperature, and mobile phase composition), these retention times are consistent and identify the compounds of interest. We use electrochemical detection, because catechols are easily oxidized, upon exposure to even the tiniest amount of oxidizing potential. The amount of electric current produced is related directly to the amount of oxidizible species in the chromatographic peak. Since early peaks are sharper, smaller concentrations of compounds can be detected the closer they appear to the solvent front. In cases where one peak is of particular interest, chromatography can be adjusted to optimize that peak for detection. The panel of catechol standards used includes DHPG (1000 pg), norepinephrine (250 pg), DOPA (1000 pg), epinephrine (250 pg), dopamine (250 pg), and DOPAC (1000 pg). Another catechol, 2,3-dihydroxybenzoic acid, is included as an internal standard.
Figure 11.3.
Chromatographic pattern of catechol standards that have undergone batch alumina extraction. A solvent front precedes individual peaks. HPLC peaks typically start out sharp and tall and become smaller and wider as retention time increases.
We hypothesized that a particular pattern of catechols would characterize Menkes disease based on the assumption of low DBH activity. Levels of dopa-mine and DOPAC were predicted to be high, and levels of norepinephrine and DHPG were predicted to be low (Fig. 11.4). This pattern was in fact the case, and the assay has been extremely useful for rapid (within hours) diagnosis of Menkes disease in the neonatal period (Figs.11.5 and 11.6; Kaler, Gahl, Berry, Holmes, & Goldstein, 1993; Schoonveld et al., 2013), when other biochemical parameters are unreliable and conventional molecular testing can take much longer (Liu, McAndrew, & Kaler, 2002). Early diagnosis is quite important since institution of treatment within 7–10 days after birth is highly desirable (Sheela, Manoj, Liu, Lem, & Kaler, 2005; Kaler et al., 2008).
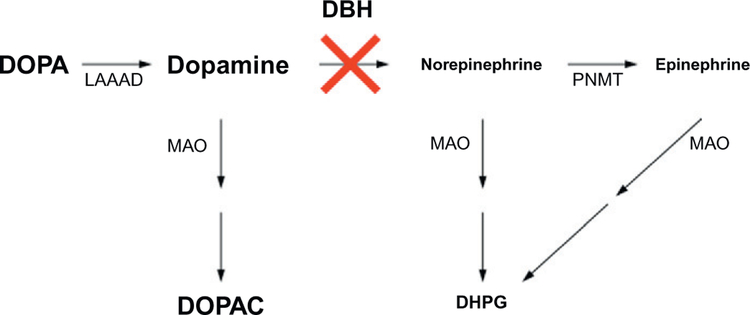
Figure 11.4.
Predicted effects of reduced DBH activity. Proximal metabolites are expected to accumulate and distal metabolites are predicted to be reduced, relative to normal.
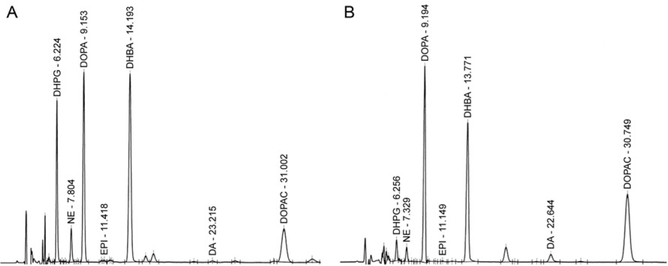
Figure 11.5.
A distinctively abnormal plasma catechol pattern exists in Menkes disease. (A) Normal catechol pattern in a newborn at risk for Menkes disease. (B) Abnormal catechol pattern in a newborn at risk for Menkes, who later was shown to have this condition based on independent clinical and molecular findings. Note the low peaks for DHPG and NE and high peaks for DOPA, DA, and DOPAC.
Figure 11.6.
Summary of the NIH experience in prospective testing of at-risk newborns for Menkes disease. Elevated plasma catechol ratios are invariably present in subjects later shown to be affected. Early diagnosis enabled institution of experimental therapy from a young age (<1 month) resulting in improved overall survival and neurodevelopmental outcome.
3. BRAIN-DIRECTED VIRAL GENE THERAPY PLUS COPPER RESCUES A MENKES MOUSE MODEL
Currently available treatment (daily subcutaneous copper injections) is not entirely effective in the majority of patients affected with Menkes disease. The mottled-brindled (mo-br) mouse recapitulates the Menkes phenotype, including abnormal copper transport to the brain owing to mutation in the murine homologue, Atp7a, and dies by 14 days of age. We documented that mo-br mice on C57BL/6 background were not rescued by peripheral copper administration and used this model to evaluate brain-directed therapies (Donsante et al., 2011). Neonatal mo-br mice received lateral ventricle injections of either adeno-associated virus serotype 5 (AAV5) harboring a reduced-size human ATP7A (rsATP7A) complementary DNA (cDNA), copper chloride, or both. AAV5-rsATP7A showed selective transduction of choroid plexus epithelia and AAV5-rsATP7A plus copper combination treatment rescued mo-br mice; 86% survived to weaning (21 days), median survival increased to 43 days, 37% lived beyond 100 days, and 22% survived to the study end point (300 days). This synergistic treatment effect correlated with increased brain copper levels, enhanced DBH activity (Fig. 11.7), and correction of brain pathology. These findings provided the first definitive evidence that gene therapy may have clinical utility in the treatment of Menkes disease.
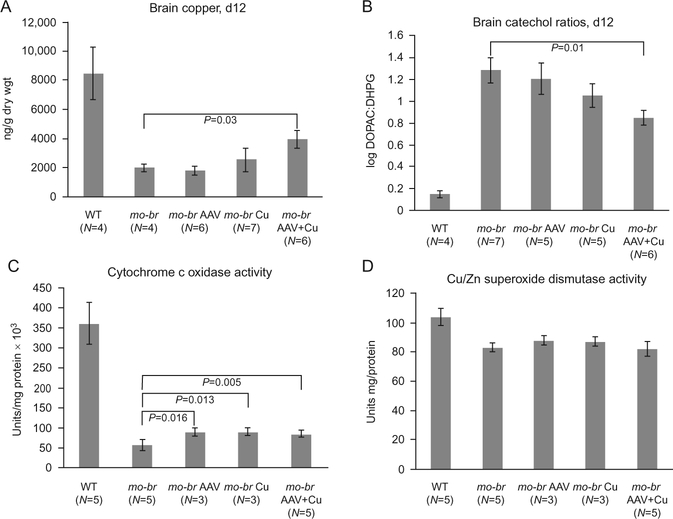
Figure 11.7.
Biochemical effects of brain-directed treatment in the mottled-brindled (mo-br) model of Menkes disease. (A) Brain copper levels at 12 days of age, by treatment category. Only AAV5 + copper (Cu) combination-treated mo-br mice showed significantly higher copper levels in comparison to untreated mo-br mice (P<0.03). (B) Brain catechol ratios at 12 days of age, by treatment category. Only AAV5+Cu combination-treated mo-br mice showed significantly lower ratios, indicative of improved dopamine-beta-hydroxylase activity (P<0.01). (C) Brain cytochrome c oxidase activity at 12 days of age, by treatment category. All treatment groups showed significantly increased activity compared to untreated mo-Br mice. (D) In contrast, the activity of Cu/Zn superoxide dismutase was ~80% of normal and treatment did not significantly enhance activity. Reproduced from Donsante et al. (2011), with permission.
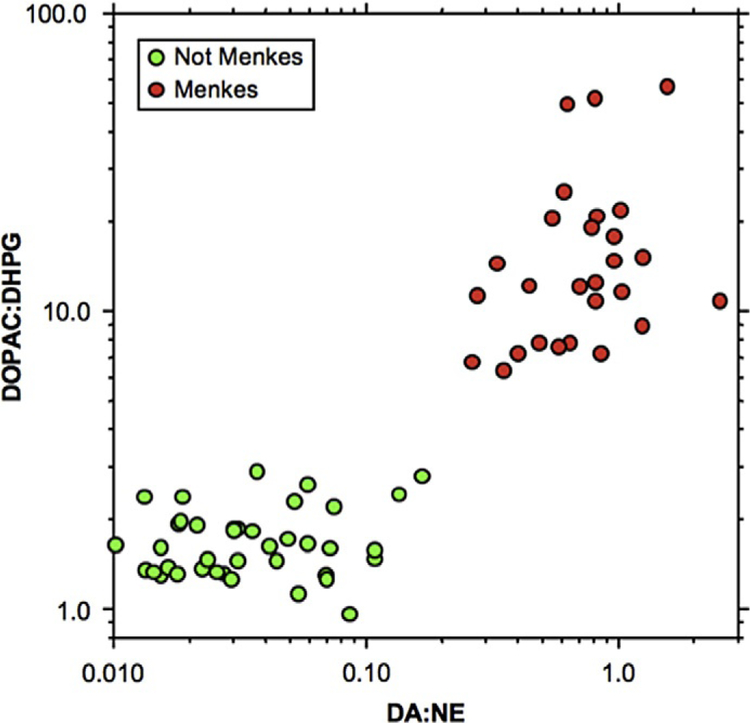
In this well-established mouse model of Menkes disease, we also tested whether systemic administration of l-threo-dihydroxyphenylserine (l-DOPS), a drug used successfully to treat autosomal recessive norepinephrine deficiency, would improve brain neurochemical abnormalities and neuropathology (Donsante, Sullivan, Goldstein, Brinster, & Kaler, 2013). At 8, 10, and 12 days of age, wild-type and mo-br mice received intraperitoneal injections of200 μg/g body weight of l-DOPS, or mock solution. Five hours after the final injection, the mice were euthanized and brains removed. We measured catecholamine metabolites affected by DBH via high-performance liquid chromatography with electrochemical detection and assessed brain histopathology. Compared to mock-treated controls, mo-br mice that received intraperitoneal l-DOPS showed significant increases in brain norepinephrine (P < 0.001) and its deaminated metabolite, DHPG (P< 0.05). The ratio of a non-beta-hydroxylated metabolite in the catecholamine biosynthetic pathway, DOPAC, to the beta-hydroxylated metabolite, DHPG, improved equivalently to results obtained previously with brain-directed ATP7A gene therapy (P<0.01). However, l -DOPS treatment did not arrest global brain pathology or improve somatic growth, as gene therapy had. We concluded that while l-DOPS crossed the BBB in mo-br mice and corrected brain neurochemical abnormalities, addition of the missing gene was necessary for rescue. Taken together, our results imply that the pattern of CSF catechols will provide a highly useful surrogate marker of treatment effect in a future AAV gene therapy clinical trial for this difficult illness.
4. CONCLUSION
Catecholamine metabolites in the plasma, brain, and CSF are affected by the copper-dependent enzyme DBH and provide highly sensitive and specific indicators for early diagnosis of Menkes disease. As new treatment approaches evolve for this illness, correction of the abnormal catechol pattern engendered by ATP7A copper transporter mutations will serve as powerful biochemical evidence of effectiveness.
ACKNOWLEDGMENTS
We thank our colleagues, Dave Goldstein and Patty Sullivan, the patients referred to NIH for catechol testing, their parents and referring physicians, and the members of the Kaler laboratory, particularly Anthony Donsante.
ABBREVIATIONS
- AAV
adeno-associated virus
- ATP7A
copper-transporting ATPase 1
- DBH
dopamine-beta-hydroxylase
- DOPA
dihydroxyphenylalanine
- DOPAC
dihydroxyphenylacetic acid
- DHPG
dihydroxyphenylglycol
- HPLC
high-performance liquid chromatography
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Biaggioni I, & Robertson D (1987).Endogenous restoration of noradrenaline by precursor therapy in dopamine-beta-hydroxylase deficiency. Lancet, 2(8569), 1170–1172. [DOI] [PubMed] [Google Scholar]
- Chelly J, Tümer Z, Tønnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, et al. (1993). Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature Genetics, 3, 14–19. [DOI] [PubMed] [Google Scholar]
- Christodoulou J, Danks DM, Sarkar B, Baerlocher KE, Casey R, Horn N, et al. (1998). Early treatment of Menkes disease with parenteral copper-histidine: Long-term follow-up of four treated patients. American Journal of Medical Genetics, 76(2), 154–164. [PubMed] [Google Scholar]
- Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, & Packman S (1995). Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. American Journal of Human Genetics, 56(3), 570–576. [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Johnson P, Jansen LA,& Kaler SG (2010). Somatic mosaicism in Menkes disease suggests choroid plexus-mediated copper transport to the developing brain. American Journal of Medical Genetics. Part A, 152A(10), 2529–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Sullivan P, Goldstein DS, Brinster LR, & Kaler SG (2013). Systemic L-threo-dihydroxyphenylserine improves neurochemical abnormalities in a mouse model of Menkes disease. Annals of Neurology, 73(2), 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Yi L, Zerfas PM, Brinster L, Sullivan P, Goldstein DS, et al. (2011). ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Molecular Therapy, 19(12), 2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Holmes CS, & Kaler SG (2009). Relative efficiencies of plasma catechol levels and ratios for neonatal diagnosis of Menkes disease. Neurochemical Research, 34, 1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Eisenhofer G, & Goldstein DS (1994). Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. Journal of Chromatography. B, Biomedical Applications, 653(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Kaler SG (1994). Menkes disease In Barness LA (Ed.), Advances in pediatrics, Vol. 41, (pp. 263–304). St. Louis: C.V. Mosby. [PubMed] [Google Scholar]
- Kaler SG (1996). Menkes disease mutations and response to early copper histidine treatment. Nature Genetics, 13, 21–22. [DOI] [PubMed] [Google Scholar]
- Kaler SG (2011). The neurology of ATP7A copper transporter disease: Emerging concepts and future trends. Nature Reviews. Neurology, 7, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Gahl WA, Berry SA, Holmes CS, & Goldstein DS (1993). Predictive value of plasma catecholamine levels in neonatal detection of Menkes disease. Journal of Inherited Metabolic Disease, 16, 907–908. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Gallo LK, Proud VK, Percy AK, Mark Y, Segal NA, et al. (1994). Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nature Genetics, 8, 195–202. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Goldstein DS, Holmes C, Salerno JA, & Gahl WA (1993). Plasmaand cerebrospinal fluid neurochemical pattern in Menkes disease. Annals of Neurology, 33, 171–175. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, et al. (2008). Neonatal diagnosis and treatment of Menkes disease. The New England Journal of Medicine, 358, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Westman JA, Bernes SM, Elsayed AM, Bowe CM, Freeman KLB, et al. (1993). Gastrointestinal hemorrhage associated with gastric polyps in Menkes disease. Journal of Pediatrics, 122, 93–99. [DOI] [PubMed] [Google Scholar]
- Liu P-C, McAndrew PE, & Kaler SG (2002). Rapid and robust screening of the Menkes disease/occipital horn syndrome gene. Genetic Testing, 6(4), 255–260. [DOI] [PubMed] [Google Scholar]
- Mercer JFB, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, et al. (1993). Isolation of a partial candidate gene for Menkes disease by positional cloning. Nature Genetics, 3, 20–25. [DOI] [PubMed] [Google Scholar]
- Møller L, Hicks JD, Holmes CS, Goldstein DS, Brendl C, Huppke P, et al. (2011). Diagnosis of copper transport disorders. Current Protocols in Human Genetics, Chapter 17; Unit 17.9, 10.1002/0471142905.hg1709s70. [DOI] [PMC free article] [PubMed]
- Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA 3rd., &Biaggioni I (1991). Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension, 18(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Schoonveld C, Donsante A, Del Gaudio D, Waggoner D, Das S, & Kaler S (2013). Prenatal diagnostic conundrum involving a novel ATP7A duplication. Clinical Genetics, 84(1), 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senard JM, & Rouet P (2006). Dopamine-beta-hydroxylase deficiency. Orphanet Journal of Rare Diseases, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela SR, Manoj L, Liu P-C, Lem KE, &Kaler SG (2005). Copper replacement treatment for symptomatic Menkes disease: Ethical considerations. Clinical Genetics, 68, 278–283. [DOI] [PubMed] [Google Scholar]
- Tang J, Robertson SP, Lem KE, Godwin SC, & Kaler SG (2006). Functional copper transport explains neurologic sparing in occipital horn syndrome. Genetics in Medicine, 8, 711–718. [DOI] [PubMed] [Google Scholar]
- Tümer Z (2013). An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Human Mutation, 34, 417–429. [DOI] [PubMed] [Google Scholar]
- Vulpe C, Levinson B, Whitney S, Packman S, & Gitschier J (1993). Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nature Genetics, 3, 7–13(Erratum, Nature Genetics (1993) 3, 273). [DOI] [PubMed] [Google Scholar]