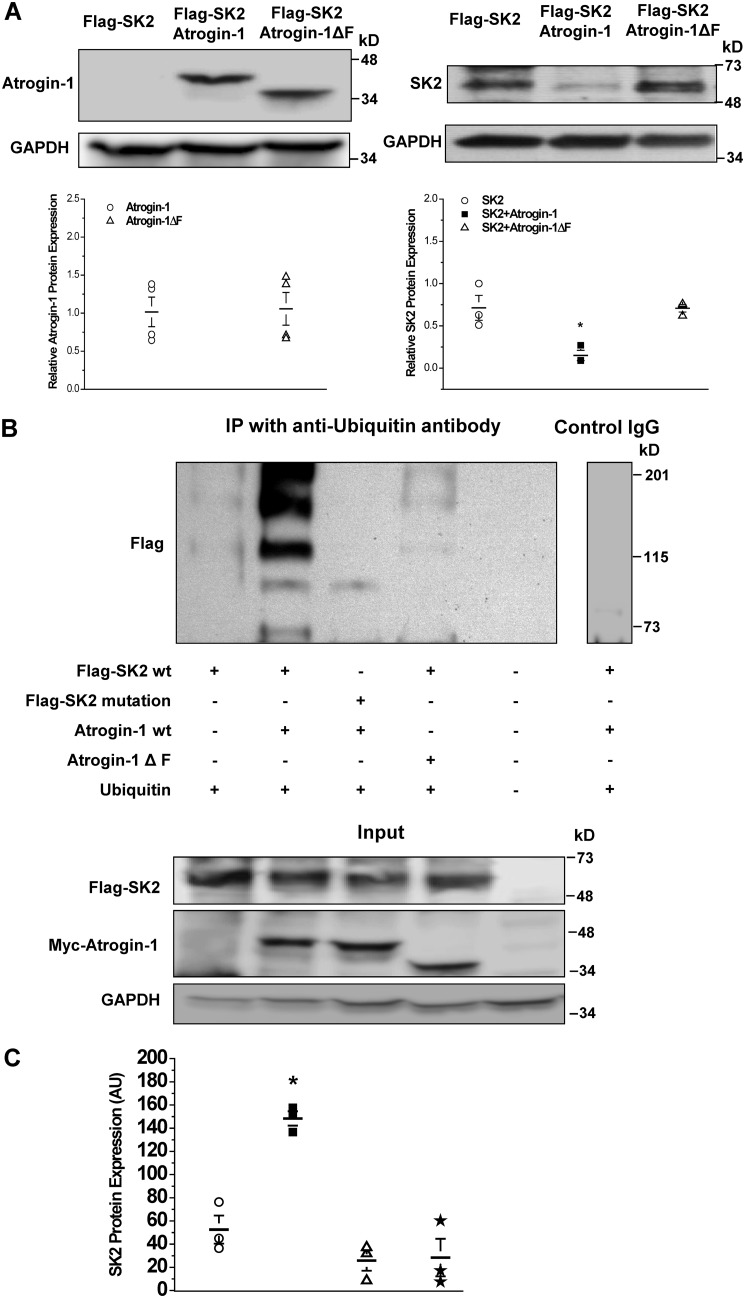
Figure 4.
Mutations in the PDZ-binding motif of SK2 prevented atrogin-1-mediated-ubiquitination and degradation of SK2. A, left panel, there was no alteration in atrogin-1ΔF protein expression compared with that of the WT atrogin-1. Right panel, co-transfection of atrogin-1 and FLAG-SK2 resulted in significant down-regulation of SK2 protein expression compared with the control (FLAG-SK2 co-transfected with empty plasmids) (*, p < 0.05, n = 3). However, co-transfection with atrogin-1ΔF had no effect on the level of SK2 expression compared with control (p = N.S., n = 3). B, top panel, immunoprecipitation with anti-ubiquitin antibody against HEK293 cell lysates with each combination of co-transfection. First lane, SK2 WT co-transfected with ubiquitin only; second lane, SK2 WT co-transfected with ubiquitin and atrogin-1; third lane, the SK2 PDZ binding motif mutant co-transfected with atrogin-1 and ubiquitin; fourth lane, SK2 WT co-transfected with atrogin-1ΔF and ubiquitin; fifth lane, lysate control; sixth lane, nonimmune IgG negative control. Ubiquitination of SK2 WT (second lane) was more substantially enhanced than all other combinations and controls. Nonimmune IgG control on the lysate of the second lane was negative (sixth lane). The experiment was independently repeated three times and produced similar results (shown in C). Bottom panel, the inputs of the tested samples as indicated in the top panel. SK2 and its mutant expression were detected by anti-FLAG antibody against the FLAG tag on the channels, and atrogin-1 and its mutant were detected by anti-myc antibody to the Myc tag fused with atrogin-1 and its mutant. C, group data for the experiments in B presented in dot scatterplots showing that significant SK2 ubiquitination occurred when SK2 WT and atrogin-1 WT were present (n = 3, p < 0.05). The positions of the lanes correspond to the position and composition of table columns in B. Data represent all anti-FLAG-reactive bands in densitometry arbitrary units (AU).