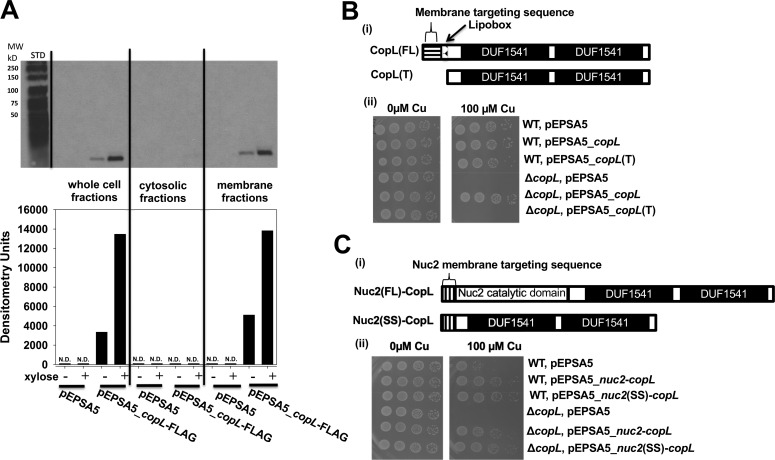
Figure 5.
CopL is membrane-associated and surface-exposed. A, monitoring CopL abundance in S. aureus whole-cell extracts, cytosolic fractions, and membrane fractions. The ΔcopL (JMB7711) mutant containing the pEPSA or pEPSA5_copL–FLAG was cultured in the absence and presence of 0.2% xylose prior to fractionation and analysis. ND, not detected. B, membrane-anchor signal sequence is necessary for CopL function. Panel i, schematic showing the CopL variants utilized. The pEPSA_copL encoded saCopL and pEPSA_copL(T) encoded for the truncated saCopL(T), which lacked the proposed N terminus export signal-sequence and lipobox. Panel ii, WT and ΔcopL strains harboring the pEPSA5, pEPSA5_copL, or pEPSA5_copL(T) were serially diluted and spot-plated on chemically defined media without and with 100 μm Cu. C, cell-surface exposure is necessary for CopL function. Panel i, schematic showing the CopL-Nuc2 chimeric variants. The truncated copL gene, lacking the proposed N-terminal export–sequence and lipobox, was fused to either the full-length nuc2 or the nuc2 membrane-anchor signal sequence and cloned into the pEPSA5 plasmid to yield the pEPSA5_nuc2(FL)-copL and pEPSA5_nuc2(SS)-copL vectors, respectively. Panel ii, WT and ΔcopL strains harboring pEPSA5, pEPSA5_nuc2(FL)-copL, or pEPSA5_nuc2(SS)-copL were serially diluted and spot-plated on chemically defined media containing 0 or 100 μm Cu.