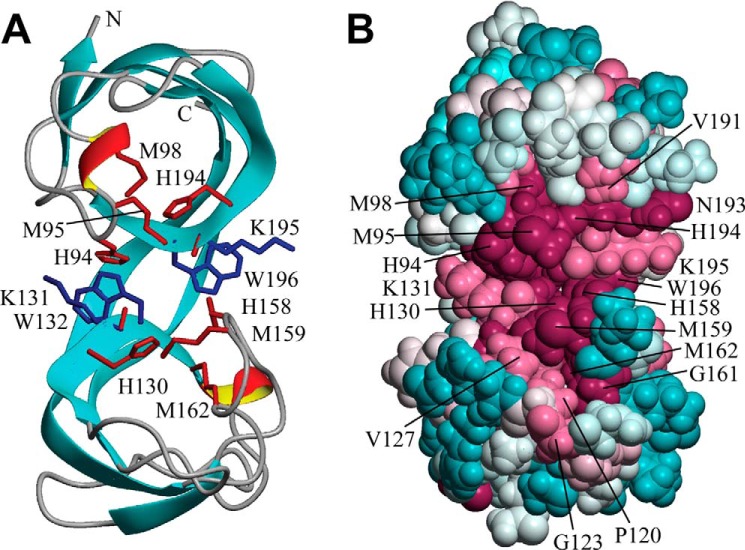
Figure 7.
Solution NMR structure of B. subtilis CopL. A, ribbon diagram of the lowest-energy conformer of B. subtilis CopL: backbone of β-strands shown in cyan, short 310-helical segments in red/yellow, and other polypeptide segments in gray; the terminal Lys-83 and Lys-205 are labeled as “N” and “C.” Side chains of Lys-131–Trp-132 and Lys-195–Trp-196 residue pairs connected by unusual cis-peptide bonds are shown in blue. Moieties presumed to be involved in coordinating Cu, side chains of His-94, Met-95, Met-98, His-130, His-158, Met-159, Met-162, and His-194, as well as carbonyls of Lys-131 and Lys-195, are shown in red. B, space-filling representation of the same conformer showing the degree of residue conservation calculated with ConSurf. Conservation scores are arranged into nine groups with the corresponding colors ranging from cyan (most variable) to burgundy (most conserved). The numbering shown here is that of the UniProt entry BSU05790.