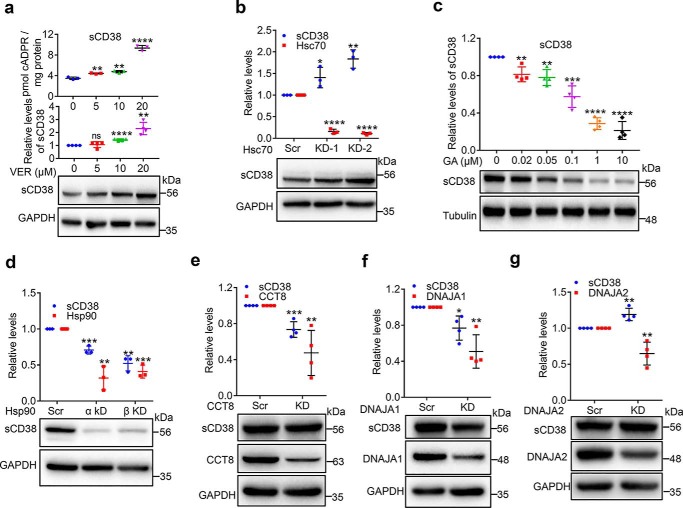
Figure 3.
Chaperones regulate the folding and proteostasis of cytosolic soluble CD38. a, sCD38-cells were treated with a series of concentrations of VER, a specific inhibitor of Hsp70s for 24 h. The protein levels of CD38, together with a housekeeping protein GAPDH were assayed by Western blotting and the cellular cADPR contents were measured by cycling assay. The representative blots (lower panel) are shown under the chart quantifying the relative expression of CD38 in at least three separate experiments (middle panel); the corresponding cADPR results are shown in the upper panel. b, sCD38-cells were transfected with Hsc70-specific or scramble siRNAs and the proteins or mRNA were analyzed by primers specific for Hsc70 or antibodies against CD38 or GAPDH. c, sCD38-cells were treated with different concentrations of geldanamycin (GA), an Hsp90 inhibitor for 3 h. The relative expression levels of CD38 were analyzed and presented as panel a. d–g, sCD38-cells were transfected with siRNAs specific for Hsp90α/β (d), CCT8 (e), DNAJA1 (f), or DNAJA2 (g) or scramble siRNA. The knockdown efficiency was evaluated by quantifying the target genes by qRT-PCR or Western blotting, and the relative expression levels of CD38 were analyzed and presented as panel a. Mean ± S.D.; n = 3 or 4; Student's t test, *, p <0.05; **, p <0.01; ***, p <0.001; ****, p <0.0001. All the protein or mRNA levels were normalized with the housekeeping genes such as GAPDH, tubulin, or β-actin and the relative levels were calculated by dividing the normalized levels by those from the control groups as described in Fig. 1.