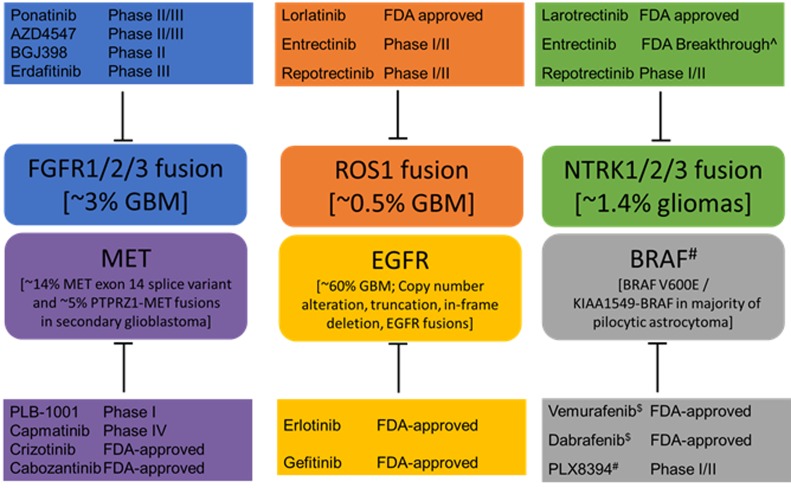
Figure 1. Genomic alterations identified in brain tumors and potential therapeutic agents.
The agents shown here have been tested in various cancer types and the highest stage of clinical trials or FDA approval for any type of tumor is indicated. Only PLB-1001 has been examined specifically in glioblastoma patients. Additional clinical studies are needed to assess the efficacy of many of these agents in the brain. $ For BRAF V600E mutants. No current FDA-approved therapy for RAF fusions. # RAF drugs which block RAF dimerization are likely to act on fusions but clinical activity not published to date. ^Breakthrough designation indicates FDA signal to expedite the development given promising preliminary signs of clinical efficacy.