Figure 1.
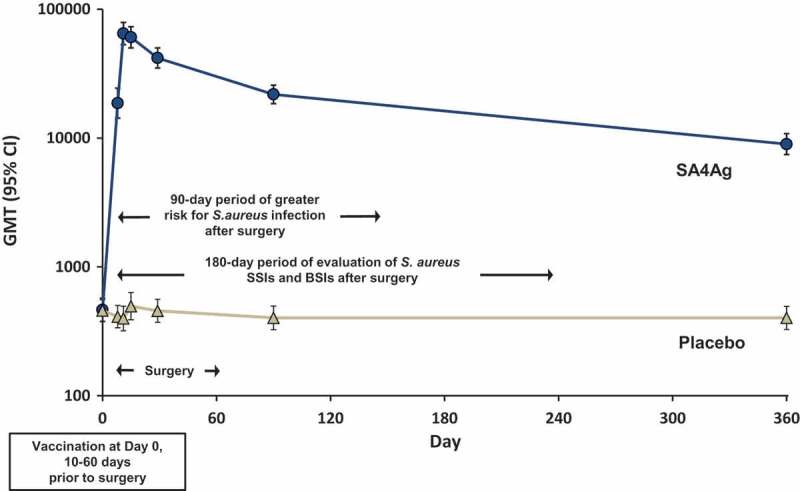
CP5 antibody levels measured by opsonophagocytic assay after SA4Ag vaccination and the risk period for S. aureus infection after surgery.
Abbreviations: BSIs = bloodstream infections; CP5 = S. aureus capsular polysaccharide serotype 5; GMT = geometric mean titer; SA4Ag = S. aureus 4-antigen vaccine; SSIs = surgical-site infections.Note: Graph represents GMTs (95% CI) for CP5 in healthy adult subjects 18 through 64 years of age. Arrows illustrate the window of time for vaccination, surgery, maximum risk of infection, and efficacy endpoint evaluation in patients included in STRIVE.Reprinted from “Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study”, Frenck RW, Creech CB, Sheldon EA, et al. Vaccine; 2017:375–384, with permission from Elsevier.
