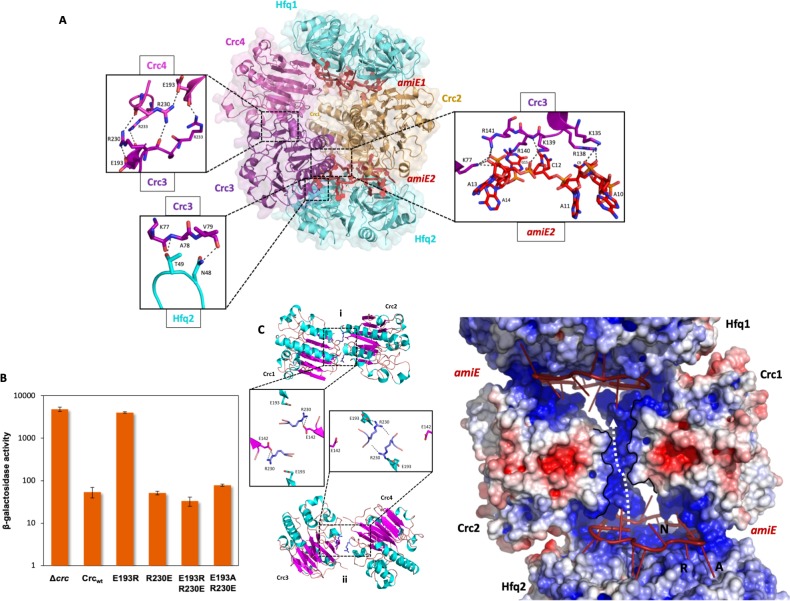
Figure 4. Model of the 2:4:2 Hfq/Crc/RNA complex and validation of interactions.
(A) Atomic model of the 2:4:2 Hfq/Crc/RNA complex. The insets show additional Hfq-Crc, Crc-Crc and Crc-RNA interactions not present in the 2:2:2 complex. The Crc3-4 dimer is formed by only one interface, with an R230-R230 interaction at the core, which globally overlaps with the dimer interface of the Crc1-Crc2 dimer (top left inset). Only one of two RNA-binding patches is presented to amiE6ARN in the Crc3-4 dimer, yet exploited more extensively (right inset). A small interface is formed between Crc3-4 and Hfq. Crc dimer in yellow, amiE6ARN RNA in red, Hfq hexamers in cyan, extra Crc dimer in magenta and purple. (B) Translational regulation of an amiE::lacZ reporter gene by Crc variants, as described in Figure 3B. The results represent data from two independent experiments and are shown as mean and range. (C) Two distinct dimeric Crc species are observed in the three complexes solved by cryoEM. i: The self-complementary interaction of the 2:2:2 core complex. ii: In the 2:4:2 complex, an alternative dimer is formed, showing a twisted dimer interface and more open configuration, with Arg230 serving as a dynamic hinge (bottom). (D) An electropositive half-channel runs along the dimer interface of the Crc1-2 dimer, and in the context of the Hfq/Crc/RNA assembly it could potentially serve as a conduit for RNA (dotted white arrow; see Figure 5). The A, R, and E RNA interaction sites of Hfq are annotated.