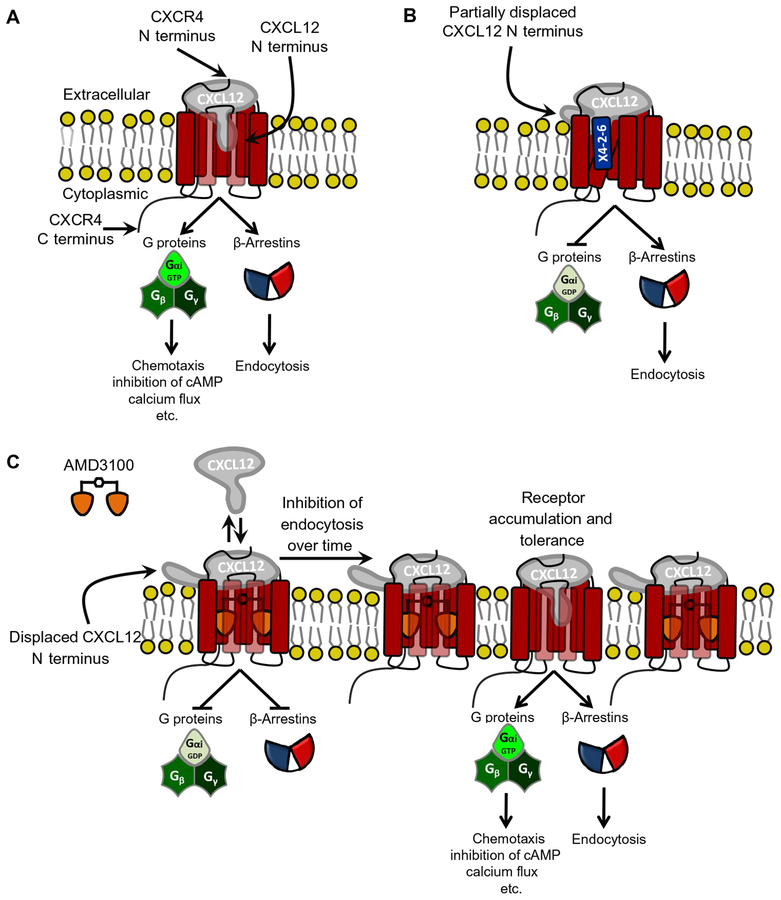
Fig. 5. Proposed model for the mechanism of biased antagonism and development of tolerance to unbiased antagonists.
(A) The current paradigm of CXCL12-mediated CXCR4 signaling suggests that the CXCL12 N terminus and N-loop insert into the CXCR4 transmembrane helical bundle, whereas the receptor N terminus binds to the globular domain of the chemokine. This leads to the activation of CXCR4 and subsequent G protein signaling, BA1/2 recruitment, and receptor endocytosis. (B) Our data suggest that X4-2-6 binds to CXCL12 and CXCR4 to form a ternary complex and displaces the extreme N-terminal portion of CXCL12 away from the transmembrane helical bundle of CXCR4. Thus, X4-2-6 functions as a biased antagonist by inhibiting G protein signaling but not BA1/2 recruitment to CXCR4. (C) In contrast, AMD3100 displaces the entire CXCL12 N terminus to inhibit all CXCR4 signaling. Over time, the inhibition of BA1/2 and the subsequent endocytosis result in the accumulation of CXCR4 on the cell surface, CXCL12 binding to the receptor, and the development of tolerance to AMD3100. GDP, guanosine diphosphate.