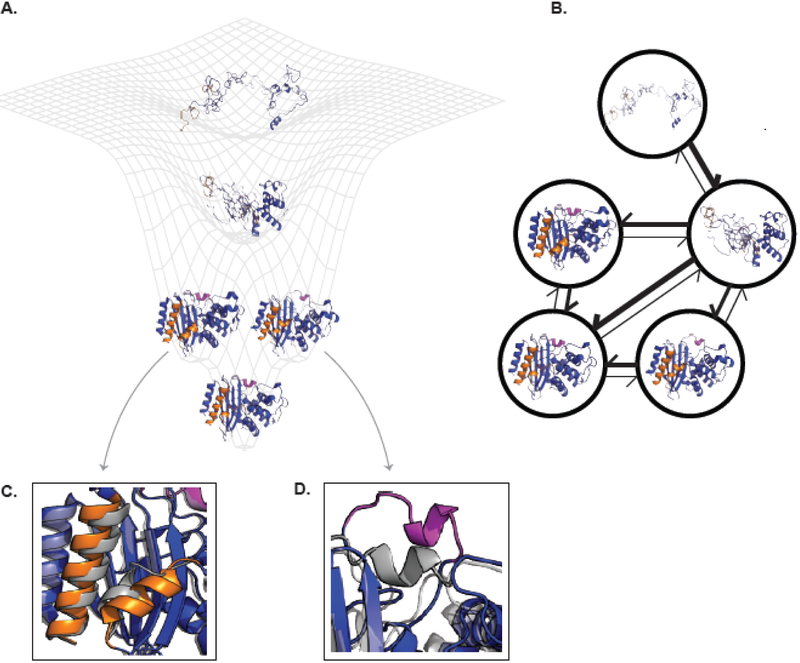
Figure 1. The connection between energy landscapes, MSMs, and protein shape-shifting.
(A) A simplified energy landscape for TEM β-lactamase. The ground state (lowest energy, highest probability state) is represented by an apo crystal structure (PDB ID 1JWP) and is shown in the bottom minima. The next two highest energy excited states each have a different cryptic pocket. The conformation on the left comes from a ligand-bound crystal structure where helices 10 and 11 (orange) have separated (PDB ID 1PZO). The structure on the right comes from computer simulations that uncovered the opening of the omega-loop (pink). The next highest energy state is a folding intermediate where the alpha-helical domain is folded while the alpha-beta domain is unfolded. The highest energy (and lowest probability) structure shown is the unfolded state. (B) The corresponding MSM for TEM β-lactamase. Each node corresponds to one of the structural states from A. The weight of the arrows is related to the probability of transitioning between the two states connected by the arrow. The same coloring is used as in (A). (C) and (D) show enlarged views of the orange and pink cryptic pockets, respectively. Each is overlaid on the apo crystal structure (gray) to highlight how the protein’s conformation has changed. Abbreviation: MSM, Markov state model.