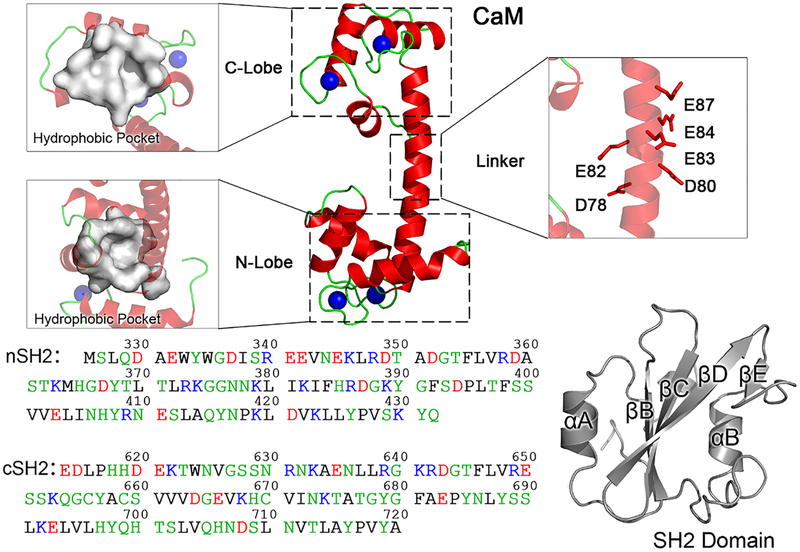
Figure 1.
Structures and sequences of CaM, nSH2, and cSH2 domains in the p85α subunit. CaM is a symmetric molecule, with two lobes (N- and C-lobe) connected by a flexible linker. The N- and C-lobes each have a hydrophobic pocket, and the linker is populated with residues with negative charges. The nSH2 and cSH2 domains share similar overall structures with a β-sheet bundle surrounded by two α-helixes, but have different amino acid sequences. In the protein sequences, hydrophobic, polar/glycine, positively charged, and negatively charged residues are colored black, green, blue, and red, respectively.