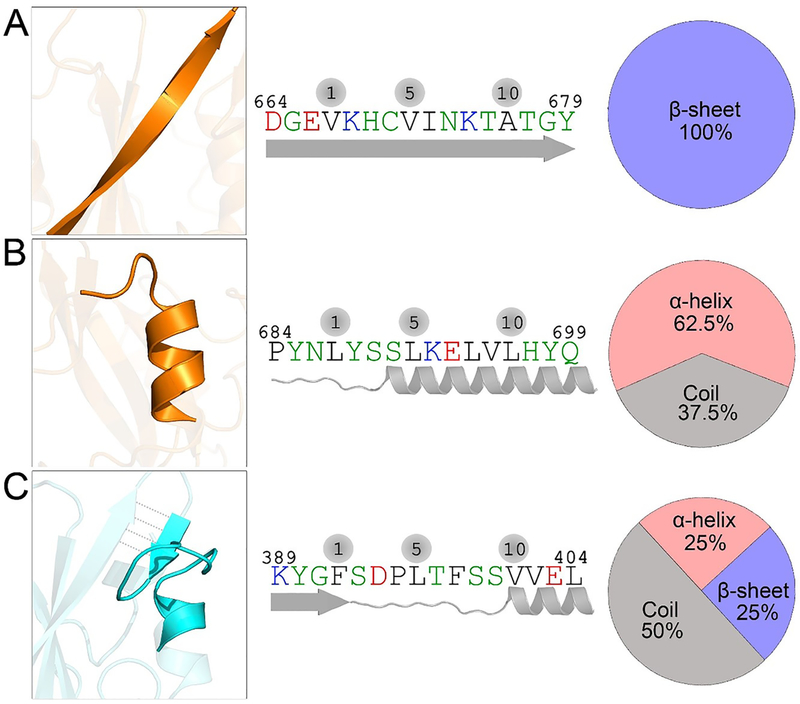
Figure 3.
CaM-binding motifs in nSH2 and cSH2 domains. (A) One CaM-binding motif in the cSH2 domain at residues 664–679 exhibits a 100% β-sheet structure, and (B) another at residues 684–699 adopts a dominant α-helix conformation. (C) The single CaM-binding motif in the nSH2 domain at residues 389–404 has mixed α-helix, random coil, and β-sheet structures.