Figure 2.
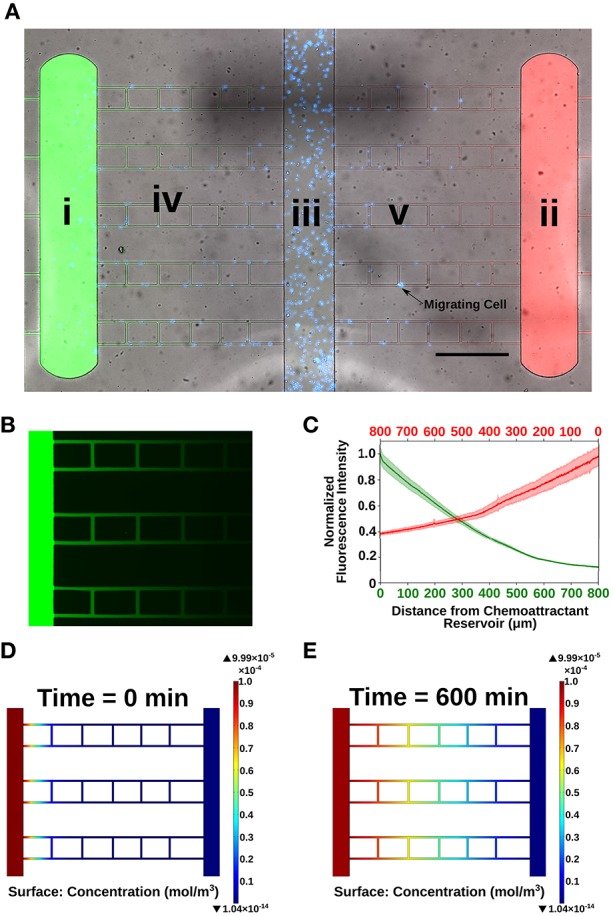
Characterization of microfluidic competitive chemotaxis-chip (μC3). (A) Microfluidic device designed to measure neutrophil migratory decision-making in a competitive chemoattractant environment. (i) Chemoattractant reservoir for end target chemoattractant (fMLP [10 nM], green). (ii) Chemoattractant reservoir for intermediary chemoattractant (LTB4 [100 nM], red). (iii) Central loading channel for neutrophils (blue). (iv) Migration channels for cells to migrate from the central loading channel to the chemoattractant reservoirs (10 × 10 μm). A linear gradient is formed along the length of the migration channels within 15 min. of priming chemoattractants into the reservoirs. (v) Ladder maze designed to measure directional chemotaxis of neutrophils. Chemoattractant gradient within ladder rungs 10-fold less than in straight migration channels. 10X Bright-field, FITC, TRITC-merged image taken in Nikon TiE. Scale bar = 500 μm. (B) Image of experimental chemoattractant gradient (green) with FITC-conjugated dextran (MW = 10,000 Da) to illustrate formation of linear gradient within the microfluidic device. (C) Quantification of the dual gradients formed within the microfluidic device. Slopes of gradients for fMLP (MW = 438 Da) and LTB4 (MW = 336 Da) are equal (within 5%) (n = 10 separate linear channels). (D,E) COMSOL finite element modeling of chemoattractant gradient over the duration of the experiment (600 min). A gradient is formed within the migration channels from the chemoattractant reservoir to the central loading channel and stays stable throughout the duration of the experiment.
