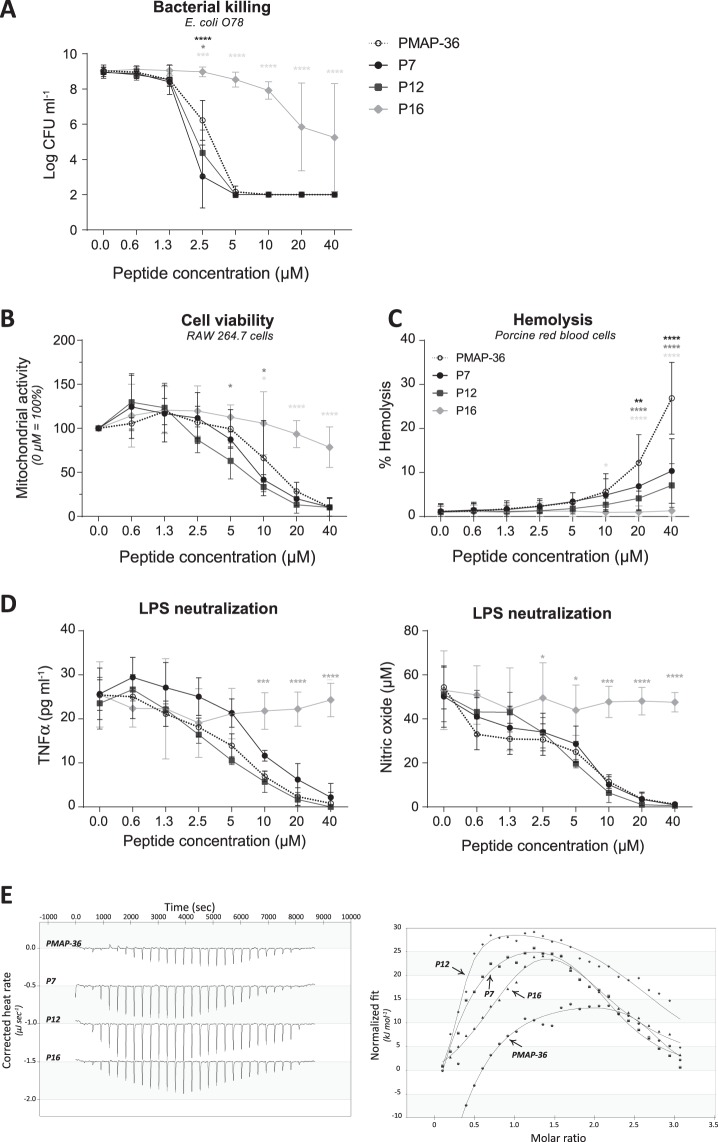
Figure 3.
Antibacterial and immunomodulatory effects of PMAP-36 are not affected by deletion of the first 11 N-terminal amino acids. Bacterial killing of PMAP-36 and analogs with N-terminal deletions were tested for their E. coli O78 killing capacities (A). Cytotoxic effects of the peptide analogs to RAW264.7 cells were determined using WST-1 reagent, indicating cell viability. No peptide control was set to 100% cell viability (B). Hemolytic effects on porcine red blood cells were determined by heme release. The positive control, 0.2% Triton was set to 100% lysis and no peptide control as background lysis (C). The PMAP-36 analogs were tested for their ability to neutralize LPS O111:B4. TNFα and NO production were used as measures for cell activation (D). Thermodynamic binding of peptides and LPS O111:B4 was measured with isothermal calorimetry (ITC). Every 300 seconds, 1.96 μl peptide solution (200 μM) was titrated into 164 μl LPS solution (25 mM). Heat evolved was measured (left panel) and normalized integrated heat was plotted against the molar ratio between LPS and the peptides (right panel). Two independent models were used to fit the data and calculate Kd (μM), the amount of peptide that binds to LPS (n), ΔH (kJ mol−1) and −TΔS (kJ mol−1). Experiments (N = 2) were corrected for heat change of dilution (buffer into buffer titration) and averaged before plotted and model fitting (E). Data is plotted as average +/− s.d. with N = 3–7 (A), N = 5–7 (B,C) and N = 3 (D). Samples were compared to PMAP-36 full-length peptide in the same concentration, using two-way ANOVA with the Bonferroni post-hoc test. (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001;****p ≤ 0.0001; black – P7; dark gray – P12; light gray – P16).