Abstract
BP-1-102, a novel inhibitor of signal transducer and activator of transcription 3 (STAT3), exhibits significant antitumor effects in several malignancies in vitro and in vivo. However, its role in gastric cancer (GC) remains to be elucidated. In the present study, the effect and potential molecular mechanisms of BP-102 in human GC cell lines were investigated. The results showed that BP-1-02 dose-dependently inhibited the proliferation of AGS cells, whereas it had little effect on HGC-27 cells. Flow cytometric analysis indicated that BP-1-102 induced apoptosis, but had minimal effect on cell cycle distribution. In addition, cells treated with BP-1-102 demonstrated markedly suppressed migration and invasion capacities. Western blot analysis revealed that BP-1-102 inhibited the phosphorylation of STAT3 and its target genes, including c-Myc, cyclin D1 and survivin, in a time- and dose-dependent manner. Furthermore, it was found that BP-1-102 induced the phosphorylation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase (MAPK) and inhibited the activation of extracellular signal-related kinases. Taken together, these results demonstrated that BP-1-102 may be a potent antitumor agent that acts through modulating the STAT3 and MAPK signaling pathways in GC cells.
Keywords: BP-1-102, gastric cancer, target therapy, signal transducer and activator of transcription 3 pathway, mitogen-activated protein kinase pathway
Introduction
Gastric cancer (GC) is one of the most common causes of cancer-associated mortality worldwide and is a major health problem in China with an increasing incidence and mortality rate according to a survey in 2015 (1,2). Although advances have been made in the detection and clinical treatment of GC in previous decades, marked variation in survival rates are found between patients diagnosed at different tumor stages. Patients diagnosed during the later stage often have metastatic disease and are no longer able to receive surgical treatment, leaving chemotherapy as the only available option (3). However, chemotherapy is often associated with a low response rate, high toxicity and drug resistance in a large number of patients (4–6). Therefore, there is an urgency for identifying and developing novel compounds to optimize therapeutic options and improve the prognosis of patients with GC.
Signal transducer and activator of transcription 3 (STAT3) is the most studied member of the STAT family and is constitutively activated in various malignancies, including GC (7). Under normal physiological conditions, cells exhibit transient STAT3 phosphorylation, which lasts for only a relatively short period of time; however, once the tumor-related signaling pathways are dysregulated, this process becomes constitutive (8). Following activation, STAT3 undergoes phosphorylation-induced dimerization, nuclear translocation and binding to its target genes, leading to the transcriptional activation of downstream target genes that regulate tumor cell proliferation and progression (3,9). There is substantial evidence demonstrating that the phosphorylation of STAT3 is abnormally activated in GC and that the constitutive activation of STAT3 is positively correlated with a poor prognosis and metastasis, indicating that it may serve as a negative prognostic factor (7,10–12). As a consequence, inhibitors targeting the activation of STAT3 offer promise in suppressing cancer proliferation and mobility and are being widely investigated in GC and several other types of cancer that contain activated STAT3 (13,14). However, the majority of STAT3 inhibitors fail to demonstrate satisfactory ability to suppress tumor growth and/or have high toxicity (15). Therefore, it remains important to identify novel inhibitors of STAT3 activation that are effective in treating GC, while producing minimal side effects.
In addition, the dysregulation of mitogen-activated protein kinase (MAPK) signaling is closely associated with cell growth, progression and apoptosis. The three most widely studied MAPKs are extracellular signal-related kinases (ERKs), p38 MAPKs and c-Jun NH2-terminal kinases (JNKs) (16). Increasing evidence suggests that the activation of ERK, p38 and JNK MAPK signaling is involved in cancer initiation and progression, indicating that they may be promising therapeutic targets (17,18).
BP-1-102 was designed as a STAT3 inhibitor and is an analog of S3I-201, which functions by disrupting STAT3 homodimerization (19). BP-1-102 inhibits the STAT3 Src homology 2 (SH2) domain, binds STAT3 with an affinity of 504 nM, and disrupts STAT3:STAT3 complex formation with a half maximal inhibitory concentration (IC50) of 6.8 µM, which is a notable improvement from that of S3I-201 (IC50=86 µM); however, BP-1-102 has minimal or no effect on other STATs, including STAT1 and STAT5 (20,21). The antitumor effect of BP-1-102 has been evaluated in human pancreatic, breast, prostate, liver and lung cancer in vitro, and in human breast and non-small cell lung tumor xenografts in vivo through either tail vein or oral administration (20,21); however, its role in GC has not been reported. Therefore, in the present study, in vitro experiments were conducted to investigate whether BP-1-102 exerts an antitumor effect on GC cells with constitutively activated STAT3 and to examine the molecular mechanisms involved.
Materials and methods
Cell lines and culture conditions
Five human gastric cancer cell lines (AGS, HGC-27, MKN28, MGC803 and SGC7901) were obtained from the Institute of Cellular Biology (Chinese Academy of Science, Shanghai, China) and cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Inc.) in a humidified 37°C incubator with 5% CO2.
Reagents and antibodies
The novel STAT3 inhibitor BP-1-102 was obtained from Selleck Chemicals, LLC (Houston, TX, USA) and dissolved in sterile dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and stored at −20°C. The primary antibodies for STAT3 (cat. no. ab68153, monoclonal, raised in rabbit, 1:2,000), phosphorylated (p-)STAT3 (Y705; cat. no. ab76315, monoclonal, raised in rabbit, 1:5,000), JNK (cat. no. ab208035, monoclonal, raised in rabbit, 1:1,000), p38 MAPK (cat. no. ab170099, monoclonal, raised in rabbit, 1:1,000), p-JNK (Y185/Y185/Y223; cat. no. ab76572, monoclonal, raised in rabbit, 1:5,000) and p-p38 MAPK (T180/Y182; cat. no. ab195049, monoclonal, raised in rabbit, 1:1,000) were purchased from Abcam (Cambridge, UK). The antibodies against p44/42 MAPK (ERK1/2; cat. no. 4695, monoclonal, raised in rabbit, 1:1,000), p-p44/42 MAPK (p-ERK1/2, T202/Y204; cat. no. 4377, monoclonal, raised in rabbit, 1:1,000), c-Myc (cat. no. 9402, polyclonal, raised in rabbit, 1:1,000), cyclin D1 (cat. no. 2922, polyclonal, raised in rabbit, 1:1,000), survivin (cat. no. 2803, polyclonal, raised in rabbit, 1:1,000), cleaved-PARP (c-PARP, cat. no. 5625, polyclonal, raised in rabbit, 1:1,000), cleaved-caspase 3 (c-caspase 3, cat. no. 9661, polyclonal, raised in rabbit, 1:1,000) and BIM (cat. no. 2933, polyclonal, raised in rabbit, 1:1,000) were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). GAPDH (cat. no. HRP-60004, 1:1,000) and HRP-conjugated secondary antibodies (cat. no. SA00001-2, 1:5,000) were purchased from ProteinTech Group, Inc. (Wuhan, China).
Cell viability assay
The AGS (3×103 cells/well) and HGC-27 (2×103 cells/well) cells were seeded into 96-well plates, exposed to DMSO vehicle (1 µM) or various concentrations of BP-1-102 (2, 4 and 6 µM in 1 µM DMSO). The maximum final concentration of DMSO was ≤0.1% in the cell culture medium. Following incubation for 24, 48 and 72 h at 37°C, a Cell Counting Kit-8 (CCK8; Dojindo Molecular Technologies, Inc., Kumamato, Japan) was used to assess cell viability following the manufacturer's protocol, and the absorbance at a wavelength of 450 nm was measured using a microplate enzyme-linked immunosorbent assay reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Colony formation assay
The AGS (5×102 cells/well) and HGC-27 (8×102 cells/well) cells were seeded in 6-well culture plates, treated with different concentrations of BP-1-102 (2, 4 and 6 µM in 1 µM DMSO) or DMSO vehicle (1 µM). Following culture for ~14 days, the colonies were fixed with 95% ethanol, stained with 0.1% crystal violet for 30 min and washed with phosphate-buffered saline, following which colony numbers were counted using an inverted microscope (magnification, ×200; Zeiss GmbH, Jena, Germany).
Flow cytometry
Apoptosis was evaluated using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Detection kit (BD Biosciences, San Jose, CA, USA). The AGS cells (2×105 cells/well) were seeded into 6-well plates and incubated overnight, following which the cells were treated with the different concentrations of BP-1-102 for 8 h. The cells were then harvested and resuspended in 500 µl of 1X binding buffer solution, incubated with Annexin V-FITC (5 µl) and PI (5 µl) at 4°C for 15 min. Subsequently, the samples were analyzed within 1 h by flow cytometry (BD Biosciences) and BD CellQuest Pro software (version 2.0, BD Pharmingen; BD Biosciences).
For cell cycle analysis, the BP-1-102-pretreated cells were trypsinized, fixed in 75% ethanol, incubated at 4°C overnight, and then centrifuged at 800 × g for 5 min at room temperature. The cells were then re-suspended in PI and RNase A solution for 30 min at room temperature in the dark and evaluated using flow cytometry (BD Biosciences) and BD CellQuest Pro software (BD Pharmingen; BD Biosciences) within 1 h.
Transwell assay for migration and invasion
For the migration and invasion assays, the cells were pre-exposed to BP-1-102 (6 µM in 1 µM DMSO) or DMSO vehicle (1 µM) for 8 h and 24-well Transwell® plates with 8-µm pore polycarbonate filters (Costar; Corning Incorporated, Corning, NY, USA) were used. The cells were harvested in serum-free RPMI-1640 medium at a density of 5×104 cells in 200 µl and seeded into the upper chambers, which had either been coated with Matrigel (BD Biosciences) or left uncoated. Subsequently, 700 µl of RPMI-1640 complete medium was added to the lower chambers. Following incubation for 18 h, the cells that had penetrated the membrane into the lower chamber were fixed with 95% ethanol and stained with 0.1% crystal violet. Images were then captured with a light microscope under ×200 magnification.
Western blot analysis
The cells (5×105 cells/well) were cultured in six-well plates. Following treatment with BP-1-102 (6 µM) for various durations (0, 3, 5 and 8 h) or at various concentrations of BP-1-102 (0, 2, 4 and 6 µM) for 8 h, the cells were harvested and quantified as previously described (22). Samples containing 20 µg of protein were subjected to electrophoresis on 10 or 12% SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membranes were blocked using blocking buffer (10% skimmed milk in Tris-buffered saline containing 0.1% Tween-20) for 1 h, incubated with primary antibodies overnight at 4°C, and then incubated with anti-rabbit HRP-conjugated secondary antibodies. Finally, the immunoreactive protein bands were visualized using an enhanced chemiluminescence western blotting kit (Bio-Rad Laboratories, Inc.) following the manufacturer's protocol.
Statistical analysis
Experimental data from experiments conducted in triplicate are presented as the mean ± standard deviation. Statistical analyses between two groups were performed using Student's t-test, and multiple comparisons were made using one-way analysis of variance followed by Dunnett's test (GraphPad Prism 5.01; GraphPad Software, Inc., San Diego, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
BP-1-102 inhibits the growth of AGS cells
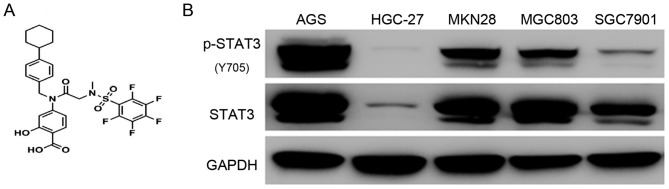
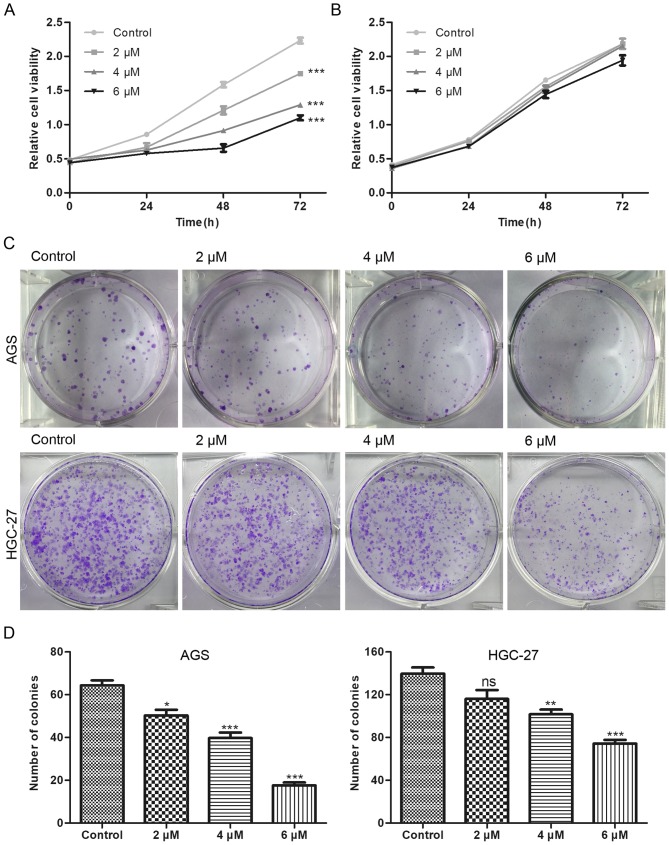
The chemical structure of BP-1-102 is shown in Fig. 1A. To evaluate the antitumor effect of BP-1-102, five GC cell-lines (AGS, HGC-27, MKN28, MGC803 and SGC7901) were used to examine the expression status and the phosphorylation level of STAT3. The AGS cells exhibited a high level of STAT3 Y705 phosphorylation, which is the most studied activated-form of STAT3, whereas the HGC-27 cells exhibited the lowest expression profile (Fig. 1B). Based on these results, the AGS and HGC-27 cell lines were selected for subsequent experiments. The AGS and HGC-27 cells were exposed to various concentrations of BP-1-102 to evaluate its effect on the proliferation of GC cells using a CCK8 assay. Compared with the control group, BP-1-102 treatment dose-dependently suppressed the proliferation of AGS cells (Fig. 2A) but had no such inhibitory effect on HGC-27 cells (Fig. 2B). Furthermore, the results of the colony formation assays showed that the BP-1-102-treated AGS cells formed smaller and fewer colonies compared with those in the control group. BP-1-102 was less effective towards HGC-27 cells than AGS cells (Fig. 2C and D). These results indicated that BP-1-102 exerted a tumor suppressive role in GC cells lines and that this effect was enhanced by high expression levels of p-STAT3 (Y705). Therefore, the AGS cell line was selected for subsequent experiments.
Figure 1.
Expression status of STAT3 in GC cell lines. (A) Chemical structure of BP-1-102. (B) Constitutive expression of STAT3 and p-STAT3 (Y 705) in five GC cell lines (AGS, HGC-27, MKNK28, MGC803 and SGC7901). STAT3, signal transducer and activator of transcription 3; p-, phosphorylated.
Figure 2.
Inhibitory effects of BP-1-102 on the proliferation of AGS cells. Cell viability, determined by Cell Counting Kit-8, of (A) AGS and (B) HGC-27 cells treated with different concentrations of BP-1-102 for 72 h. (C) AGS and HGC-27 cells were treated with BP-1-102 at 2–6 µM and cultured for ~14 days until the colonies were visible. (D) Histograms of the number of cells in each treatment group in AGS (left) and HGC-27 (right) cells. Data were obtained from three independent experiments. *P<0.05, **P<0.01 and ***P<0.001, vs. control. ns, not significant.
BP-1-102 induces apoptosis in AGS cells and has little effect on cell cycle
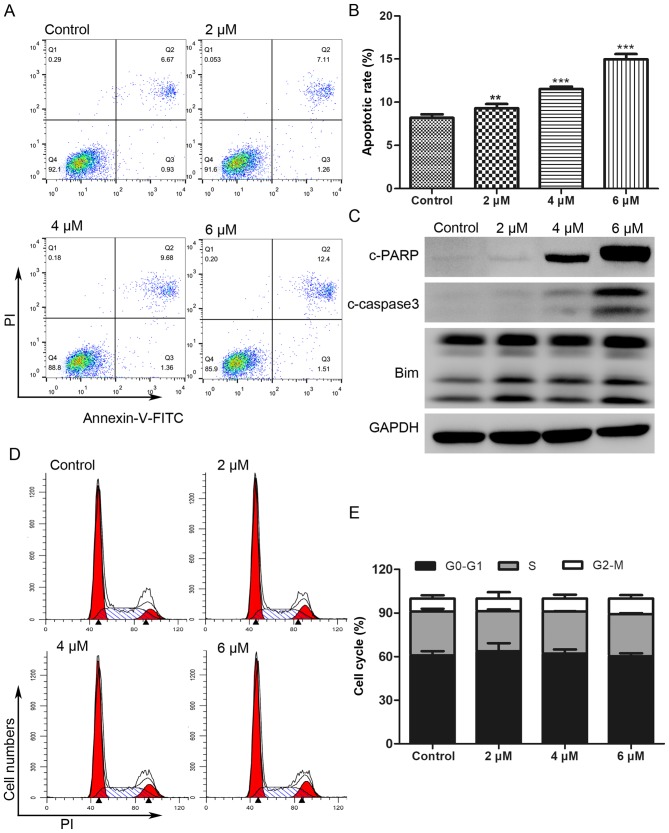
The Annexin V-FITC/PI double staining assay was performed to investigate whether the antitumor effect of BP-1-102 was associated with the induction of apoptosis. As shown in Fig. 3A and B, the proportion of apoptotic AGS cells (Annexin V-FITC positive) increased from 7.6% in the untreated control cells to 8.37, 11.04 and 13.91% following treatment for 8 h with 2, 4 and 6 µM of BP-1-102, respectively. The expression of apoptotic-related proteins was analyzed by western blotting. As shown in Fig. 3C, BP-1-102 treatment markedly increased the expression of cleaved-poly (ADP-ribose) polymerase (PARP), cleaved-caspase 3, and B-cell lymphoma 2 (Bcl-2)-interacting mediator of cell death (Bim) in a dose-dependent manner. BP-1-102 treatment had no significant influence on the AGS cell cycle distribution (Fig. 3D and E).
Figure 3.
BP-1-102 induces apoptosis in AGS cells and has little effect on cell cycle. (A) Annexin V and PI double staining of AGS cells was performed using flow cytometry to evaluate the apoptotic cells in AGS following exposure to different concentrations of BP-1-102 for 8 h. (B) Histograms of the percentage of apoptotic cells. (C) Western blot analysis of apoptosis-related proteins. GAPDH was used as a protein loading control. (D) PI staining and flow cytometric analysis of AGS cells pretreated with different concentrations of BP-1-102 for 8 h. (E) Cell cycle distribution shown as histograms. The results are representative of three independent experiments. **P<0.01 and ***P<0.001, vs. control. PARP, poly (ADP) ribose polymerase; Bim, B-cell lymphoma-2-interacting mediator of cell death; c-, cleaved; PI, propidium iodide; FITC, fluorescein isothiocyanate.
BP-1-102 inhibits the mobility of AGS cells
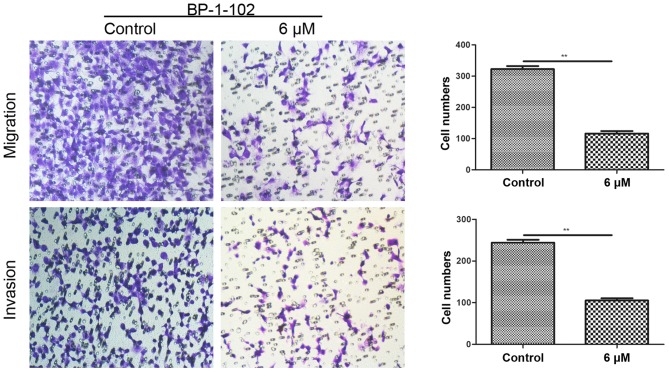
Transwell assays were conducted to assess the effect of BP-1-102 on tumor cell migration and invasion. As shown in Fig. 4, the number of penetrating cells was significantly lower in the BP-1-102-treated group (6 µM) compared with that in the control group (untreated). These data indicate the involvement of BP-1-102 in suppressing the mobility of AGS cells.
Figure 4.
BP-1-102 suppresses the migration and invasion of AGS cells. Transwell assay showed that BP-1-102 markedly attenuated the migration (without Matrigel) and invasion capacity (with Matrigel) of AGS cells. Following pre-exposure to BP-1-102 for 8 h, the cells were seeded in the top compartment of the Transwell chambers and incubated for 18 h (magnification, ×200). The numbers of migrated and invaded cells were quantified from three independent experiments. **P<0.01, vs. control.
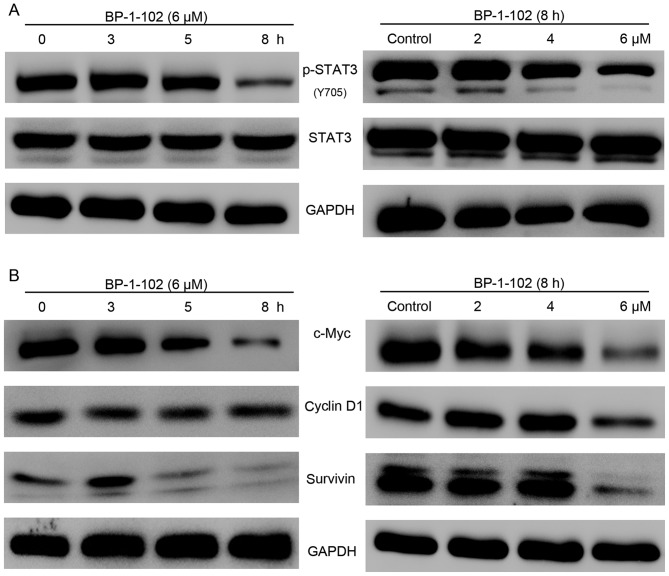
BP-1-102 inhibits the phosphorylation of STAT3 and its target genes in a time-dependent and dose-dependent manner
The previous findings demonstrated that BP-1-102 inhibited cell growth, migration and invasion ability and induced apoptosis. To gain insight into the potential mechanism of BP-1-102 activity in AGS cells, the activation of STAT3 and its target genes were examined using western blot analysis. As shown in Fig. 5A, the expression of p-STAT3 (Y705) was markedly suppressed by BP-1-102 treatment in a time- and dose-dependent manner, whereas the expression levels of total STAT3 remained unchanged. The STAT3 signaling pathway regulates cell proliferation and survival by modulating the expression of various target genes. The results showed that BP-1-102 also time- and dose-dependently downregulated the expression of c-Myc, cyclin D1 and survivin (Fig. 5B).
Figure 5.
BP-1-102 inhibits the phosphorylation of STAT3 and downregulates the expression of STAT3-regulated genes in AGS cells in a time- and dose-dependent manner. (A) Western blot analysis of p-STAT3 and STAT3 in AGS cells following treatment with 6 µM BP-1-102 for various durations (left) or with different concentrations of BP-1-102 for 8 h (right). (B) BP-1-102 time-dependently (left) and dose-dependently (right) decreased the expression of c-Myc, cyclin D1 and survivin in AGS cells. The results are representative of three independent experiments. STAT3, signal transducer and activator of transcription 3; p-, phosphorylated.
BP-1-102 modulates the expression of MAPKs
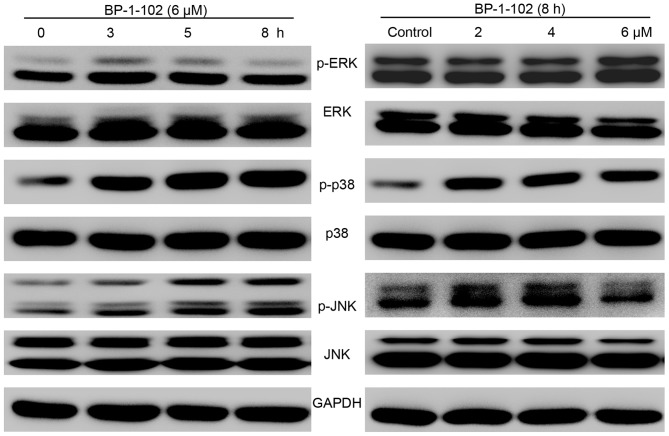
As BP-1-102 inhibited cell growth and mobility and induced apoptosis, the possible mechanisms involved were investigated by evaluating the effect of BP-1-102 treatment on the elementary activation status of MAPKs. As shown in Fig. 6, following exposure of the AGS cells to BP-1-102, the phosphorylation of p38 and JNK was upregulated, whereas the phosphorylation of ERK was decreased, in a time- and dose-dependent manner relative to the total levels of p38, JNK and ERK, which served as internal controls.
Figure 6.
Effects of BP-1-102 on MAPKs. Western blot analysis was used to evaluate the expression of MAPKs (p-ERK, ERK, p-p38, p38, p-JNK and JNK). Cells were treated with the indicated drug concentrations for different durations (right) or with indicated concentrations of BP-1-102 for 8 h (right). The results are representative of at least three independent experiments. MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase; p-, phosphorylated.
Discussion
STAT3 is pivotal in signal transduction and transcription, and regulates the transcriptional activity of downstream target genes involved in cell proliferation (c-Myc and cyclin D1), anti-apoptotic genes (Bcl-2, Bcl-extra large and survivin) and genes involved in invasion (matrix metalloproteinase-2 and −9) (23,24). Although the basal expression levels differ in tissues from different organs, STAT3 is frequently overexpressed or constitutively activated in human solid tumors compared with matched normal tissues (25). Previously, constitutively activated STAT3 was reported to occur in various types of cancer, including prostate, renal, breast, ovarian and liver cancer, and is correlated with poor overall survival rates of patients with lung cancer or GC (26–28). In particular, a previous study of GC found that the overexpression levels of p-STAT3 and total STAT3 were correlated with poor overall survival rate (29). Therefore, although the role of STAT3 in tumorigenesis remains to be fully elucidated, the inhibition of STAT3 continues to be an attractive strategy for the clinical treatment of tumors with activated STAT3.
Several strategies have been established for the inhibition of STAT3, including the inhibition of upstream activators, inhibiting the phosphorylation of STAT3 itself, and suppressing the nuclear translocation of STAT3; notably a large number of inhibitors targeting STAT3 have been identified over the last few decades (14,30). Specifically, several non-peptide STAT3-SH2 domain inhibitors, including S3I-201, STATTIC and STA-21, have been found to be effective in suppressing cell growth in vitro and in vivo (31–33). S3I-201 (NSC 74859) was effective in inhibiting the formation of STAT3 dimers (IC50=86 µM) and the expression of STAT3 target genes (c-Myc, cyclin D1 and survivin) in breast cancer (33). In liver cancer, it was found that S3I-201 was not only able to inhibit tumor growth but also significantly enhanced the antitumor effects of cetuximab and doxorubicin (34–36). STATTIC can selectively inhibit STAT3 dimerization (IC50=5.1 µM) and nuclear translocation in breast and liver cancer (31). In addition, it has been found that STATTIC enhances chemo- and radio-sensitivity in patients with head and neck squamous cell carcinoma (37,38). STA-21 was found to reduce the survival of breast carcinoma cells with hyperactivated STAT3; however, it exerted little effect on cells with a low expression of activated STAT3 (32). STA-21 has been also reported to be involved in regulating cell differentiation and inflammation (39–41). However, although present inhibitors have an acceptable antitumor effect, they are neither effective at low concentrations nor orally bioavailable. For these reasons, although substantial progress has been made, further progress is necessary, and novel inhibitors with higher efficiency, improved bioavailability and fewer side effects are required. BP-1-102 is a novel orally bioavailable STAT3 inhibitor that functions by directly interacting with STAT3 at a relatively low concentration (IC50=6.4 µM) and has been shown to have significant antitumor effects in several tumors (19); however, its potential use in GC treatment has not been investigated previously.
In the present study, cell growth, colony formation, apoptosis, cell cycle and cell mobility were evaluated in vitro to determine the effect of BP-1-102 on the biological characteristics of AGS cells. It was found for the first time, to the best of our knowledge, that BP-1-102 exhibited superior suppressive effects on the cell proliferation and colony formation capacities of AGS cells with a higher expression level of p-STAT3 (Y705) than HGC-27 cells with lower expression levels of p-STAT3 (Y705). In addition, the expression status of STAT3 and several STAT3-regulated genes were examined. The results revealed that the constitutive activation of STAT3 was inhibited by BP-1-102 in a time- and dose-dependent manner. Subsequently, the expression levels of c-Myc and cyclin D1, two proteins associated with the inhibition of cell proliferation, decreased when the cells were exposed to BP-1-102. Apoptosis assays demonstrated that the proportion of apoptotic cells increased upon incubation of the cells with BP-1-102, which can be attributed to suppression of the anti-apoptotic protein surviving (42). Additionally, several apoptotic-related proteins were measured via western blotting. When exposed to BP-1-102, the expression of cleaved-PARP, cleaved-caspase 3 and pro-apoptotic Bim were markedly increased in the AGS cells in a dose-dependent manner. However, although a significant decrease in the expression of cyclin D1, an important target in regulating cell cycle (43), was observed, the cell cycle distribution of AGS cells remained unchanged when pretreated with BP-1-102 for 8 h. In addition, based on the results of the Transwell assays, the migration and invasion capacities of AGS cells were markedly suppressed by BP-1-102 treatment. The detailed mechanisms underlying these effects of BP-1-102 on AGS cells remain to be elucidated.
The dysregulation of MAPK is critical in tumor development (18,44). ERK, the most widely studied MAPK, has been shown to be a major regulator in cell growth and the activation of p38 and JNK MAPKs are reported to be positively correlated with apoptosis (16,45,46). In the present study, BP-1-102 was found to activate JNK and p38 MAPK and to inactivate ERK in AGS cells in a time- and dose-dependent manner. The results suggest that BP-1-102 may also act as a MAPK regulator in AGS cells. However, future investigations are warranted to further examine the roles of MAPKs in the effects of BP-1-102 on growth suppression and apoptosis induction in AGS cells. There were several limitations to the present study. For example, the mechanism was based on a single cell line and in vivo experiments are required to further evaluate the antitumor effect and toxicity of BP-1-102. Further experiments, including in vivo experiments are to be performed in the future to validate its antitumor potential in GC.
In conclusion, the present study is the first, to the best of our knowledge, to demonstrate that BP-1-102 is a potent inhibitor of STAT3 signaling and a potential regulator of MAPKs in GC cells. BP-1-102 suppressed the proliferation, migration and invasion capacities of AGS human gastric cancer cells and induced their apoptosis. These findings suggest that BP-1-102 may be a potential therapeutic agent. Future in vivo experiments are warranted to further evaluate this possible approach, and to determine the efficacy and safety of BP-1-102 in treating patients with GC with hyperactivated STAT3.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81201089 and 81272676) and the Natural Science Foundation of Zhejiang province, China (grant nos. LY15H160026 and LY15H160012).
Availability of data and materials
The data used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Authors' contributions
XXJ, JT, ZQL, XFY and LST conceived and designed the study. XXJ, JT, MJW, STC, ZZX, YHW and HHW performed the data analysis. XXJ and JT wrote the paper. All the authors reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA. Chemotherapy for gastric carcinoma: New and old options. Oncology (Williston Park) 1998;12:44–47. [PubMed] [Google Scholar]
- 5.Tannock IF. Cancer: Resistance through repopulation. Nature. 2015;517:152–153. doi: 10.1038/nature14075. [DOI] [PubMed] [Google Scholar]
- 6.Kessler DA, Austin RH, Levine H. Resistance to chemotherapy: Patient variability and cellular heterogeneity. Cancer Res. 2014;74:4663–4670. doi: 10.1158/0008-5472.CAN-14-0118. [DOI] [PubMed] [Google Scholar]
- 7.Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073–1085. doi: 10.1053/j.gastro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajimoradi M, Mohammad Hassan Z, Ebrahimi M, Soleimani M, Bakhshi M, Firouzi J, Samani FS. STAT3 is overactivated in gastric cancer stem-like cells. Cell J. 2016;17:617–628. doi: 10.22074/cellj.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010;16:5380–5387. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386–1393. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- 13.Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs. 2016;25:1023–1031. doi: 10.1080/13543784.2016.1195807. [DOI] [PubMed] [Google Scholar]
- 14.Furtek SL, Backos DS, Matheson CJ, Reigan P. Strategies and approaches of targeting STAT3 for cancer treatment. ACS Chem Biol. 2016;11:308–318. doi: 10.1021/acschembio.5b00945. [DOI] [PubMed] [Google Scholar]
- 15.Lai PS, Rosa DA, Magdy Ali A, Gómez-Biagi RF, Ball DP, Shouksmith AE, Gunning PT. A STAT inhibitor patent review: Progress since 2011. Expert Opin Ther Pat. 2015;25:1397–1421. doi: 10.1517/13543776.2015.1086749. [DOI] [PubMed] [Google Scholar]
- 16.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 17.Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- 18.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: An update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 19.Page BD, Fletcher S, Yue P, Li Z, Zhang X, Sharmeen S, Datti A, Wrana JL, Trudel S, Schimmer AD, et al. Identification of a non-phosphorylated, cell permeable, small molecule ligand for the Stat3 SH2 domain. Bioorg Med Chem Lett. 2011;21:5605–5609. doi: 10.1016/j.bmcl.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resetca D, Haftchenary S, Gunning PT, Wilson DJ. Changes in signal transducer and activator of transcription 3 (STAT3) dynamics induced by complexation with pharmacological inhibitors of Src homology 2 (SH2) domain dimerization. J Biol Chem. 2014;289:32538–32547. doi: 10.1074/jbc.M114.595454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng L, Xu C, Guan Z, Su X, Xu Z, Cao J, Teng L. Galectin-1 mediates TGF-β-induced transformation from normal fibroblasts into carcinoma-associated fibroblasts and promotes tumor progression in gastric cancer. Am J Transl Res. 2016;8:1641–1658. [PMC free article] [PubMed] [Google Scholar]
- 23.Luwor RB, Stylli SS, Kaye AH. The role of Stat3 in glioblastoma multiforme. J Clin Neurosci. 2013;20:907–911. doi: 10.1016/j.jocn.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Yue P, Fletcher S, Zhao W, Gunning PT, Turkson J. A novel small-molecule disrupts Stat3 SH2 domain-phosphotyrosine interactions and Stat3-dependent tumor processes. Biochem Pharmacol. 2010;79:1398–1409. doi: 10.1016/j.bcp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang S. Regulation of STAT signaling by acetylation. Cell Signal. 2013;25:1924–1931. doi: 10.1016/j.cellsig.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong M, Wang J, Jiang N, Pan H, Li D. Correlation between p-STAT3 overexpression and prognosis in lung cancer: A systematic review and meta-analysis. PLoS One. 2017;12:e0182282. doi: 10.1371/journal.pone.0182282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu S, Li G, Wang Z, Wang Z, Chen C, Cai S, He Y. The prognostic value of pSTAT3 in gastric cancer: A meta-analysis. J Cancer Res Clin Oncol. 2016;142:649–657. doi: 10.1007/s00432-015-2023-1. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: Modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 29.Pan YM, Wang CG, Zhu M, Xing R, Cui JT, Li WM, Yu DD, Wang SB, Zhu W, Ye YJ, et al. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer. Mol Cancer. 2016;15:79. doi: 10.1186/s12943-016-0561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelain A, Mori M, Meneghetti F, Villa S. Signal transducer and activator of transcription protein 3 (STAT3): An update on its direct inhibitors as promising anticancer agents. Curr Med Chem. 2018 Jul 19; doi: 10.2174/0929867325666180719122729. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 31.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu QD, Chen W, Yan TL, Ma T, Chen CL, Liang C, Zhang Q, Xia XF, Liu H, Zhi X, et al. NSC 74859 enhances doxorubicin cytotoxicity via inhibition of epithelial-mesenchymal transition in hepatocellular carcinoma cells. Cancer Lett. 2012;325:207–213. doi: 10.1016/j.canlet.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Shen X, Xia X, Xu G, Ma T, Bai X, Liang T. NSC 74859-mediated inhibition of STAT3 enhances the anti-proliferative activity of cetuximab in hepatocellular carcinoma. Liver Int. 2012;32:70–77. doi: 10.1111/j.1478-3231.2011.02631.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson L, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Zhang C, He J, Guo Q, Hu D, Yang X, Wang J, Kang Y, She R, Wang Z, et al. STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumour Biol. 2015;36:2135–2142. doi: 10.1007/s13277-014-2823-y. [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS One. 2013;8:e54565. doi: 10.1371/journal.pone.0054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad SF, Ansari MA, Nadeem A, Zoheir KMA, Bakheet SA, Alsaad AMS, Al-Shabanah OA, Attia SM. STA-21, a STAT-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology. 2017;222:206–217. doi: 10.1016/j.imbio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Park JS, Kwok SK, Lim MA, Kim EK, Ryu JG, Kim SM, Oh HJ, Ju JH, Park SH, Kim HY, Cho ML. STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis Rheumatol. 2014;66:918–929. doi: 10.1002/art.38305. [DOI] [PubMed] [Google Scholar]
- 41.Takaishi M, Yokogawa M, Miyoshi K, Nakajima K, DiGiovanni J, Sano S. STA-21, a stat3 inhibitor, induces differentiation of normal human epidermal keratinocytes and human keratinocyte derived cell lines. J Invest Dermatol. 2010;130(Suppl):S97–S97. [Google Scholar]
- 42.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 43.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 44.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scuteri A, Galimberti A, Maggioni D, Ravasi M, Pasini S, Nicolini G, Bossi M, Miloso M, Cavaletti G, Tredici G. Role of MAPKs in platinum-induced neuronal apoptosis. Neurotoxicology. 2009;30:312–319. doi: 10.1016/j.neuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed in the present study are available from the corresponding author on reasonable request.