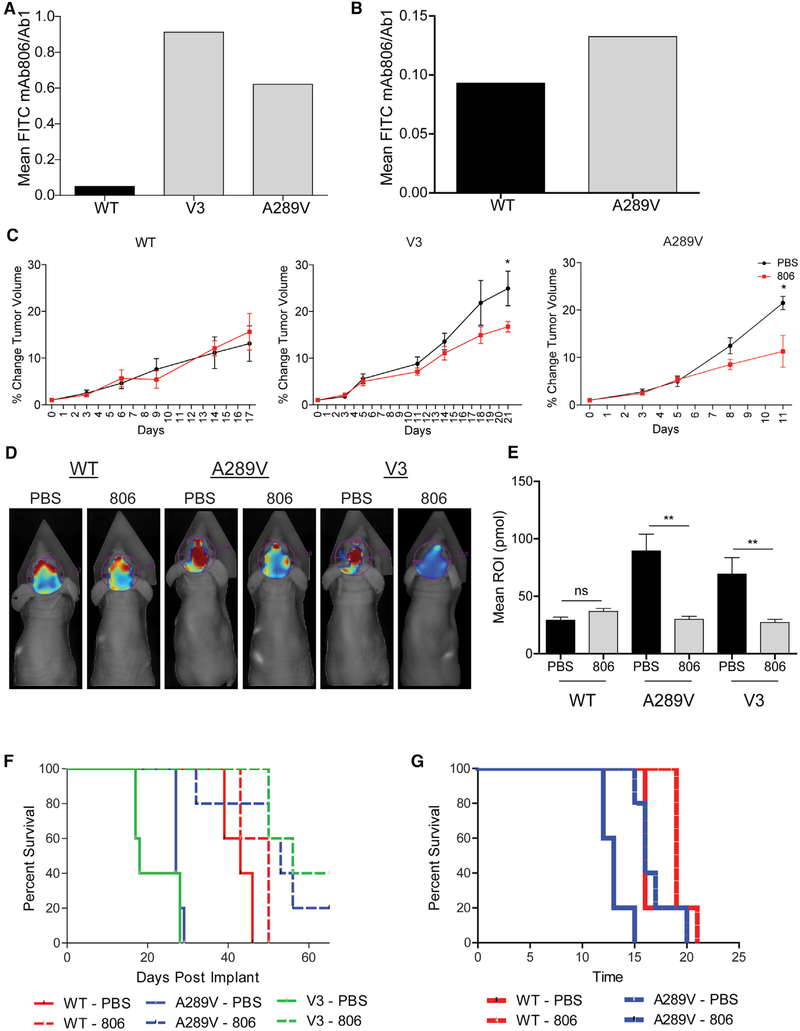
Figure 6. mAb806 as a Therapeutic Option for Patients Expressing EGFRA289V.
(A and B) Fluorescent-activated cell sorter analysis of U87 glioma cells (A) and HK281 GBM-spheres (B) expressing WT EGFR, EGFRvIII, or EGFRA289V. Serum-starved cells were incubated with either mAb806 or mAb528 (1 μg/1 × 106 cells) followed by secondary staining with a fluorescein isothiocyanate-conjugated antibody. Results are shown as mAb806 staining normalized to mAb528 (total EGFR).
(C) Mice bearing subcutaneous U87 tumors expressing WT EGFR, EGFRvIII (V3), or EGFRA289V were treated with PBS (100 μL/mouse) or mAb806 (0.1 mg/100 μL/mouse) intraperitoneally (i.p.) 3 times per week for 2 weeks once tumors reached an average of 100 mm3. Mean tumor growth after treatment is shown as a function of time.
(D) Representative images by fluorescence molecular topography (FMT) at day 23 of mice implanted intracranially with iRFP720 expressing U87 glioma cells expressing WT EGFR, EGFRvIII, or EGFRA289V and treated with PBS (100 μL) or mAb806 (1 mg/100 μL/mouse) i.p. every other day from days 0 to 14.
(E) Quantification of FMT signal intensity on day 23 post-implantation for each region of interest of mice in (D).
(F) KM survival curve of mice in (D and E).
(G) KM survival curve of mice bearing HK281 intracranial tumors as described in (D). n = 5 for each animal group.
Error bars are SEM. ns, not significant; *p < 0.05, **p < 0.01. See also Figure S7.