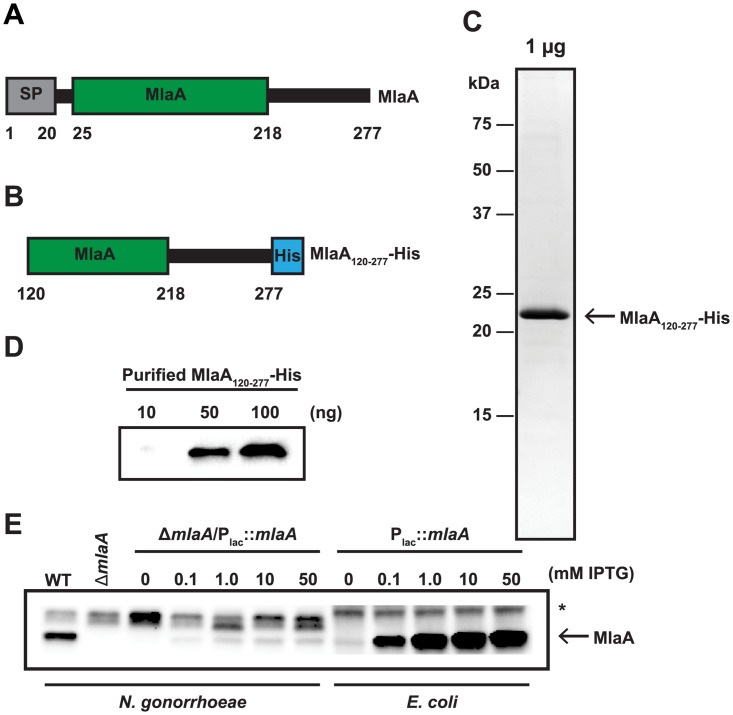
Fig 3. Purification of a truncated recombinant version of MlaA and polyclonal anti-MlaA serum validation.
(A) Representative schematic of full length MlaA with annotated MlaA domain. A signal peptide (SP) is noted by a grey rectangle. (B) Schematic of truncated MlaA used for purification with first 119 amino acids removed and a 6 × Histidine tag (His; as a blue rectangle) added to the N-terminus. Schematics are not to scale. (C) Truncated MlaA construct was overexpressed in E. coli and purified by nickel affinity chromatography in the presence of 1% Triton-X 100 detergent. Detergent was subsequently removed by incubation with Bio-Rad Bio-Beads SM-2 resin. To assess purity, 1 μg of protein was subjected to 1D SDS-PAGE and visualized by staining with Colloidal Coomassie Blue G-250. Open arrow indicates migration band of MlaA120-277. Migration of a molecular weight marker (in kDa) is indicated to the left of the gel. (D) Immunoblot evaluation of anti-MlaA antiserum. Indicated amounts of purified MlaA120-277 were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-MlaA antiserum. (E) Equivalent OD600 units of WT, isogenic ΔmlaA, and either ΔmlaA/Plac::mlaA or E. coli harboring the pGCC4-ngo2121 complementation plasmid cultured with indicated concentrations of IPTG, were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-MlaA antiserum. Open arrow indicates MlaA. Non-specific cross-reactive band is marked with an asterisk (*). OD600, optical density at 600 nm; IPTG, isopropyl β-D-thiogalactopyranoside; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.