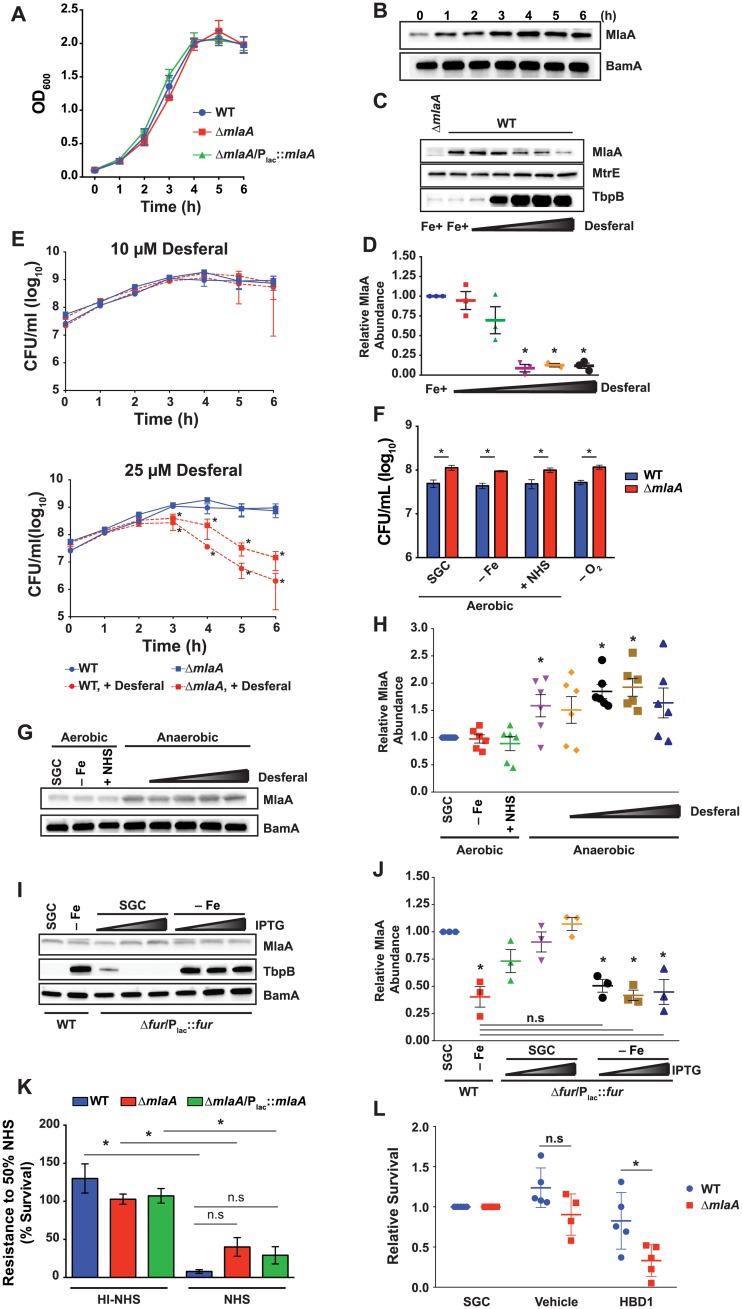
Fig 5. In vitro fitness assessments and MlaA expression profiling.
(A) WT FA1090, isogenic knockout ΔmlaA, and complementation strain ΔmlaA/Plac::mlaA were cultured aerobically in liquid medium. IPTG was added to 0.1 mM in ΔmlaA/Plac::mlaA cultures. Bacterial growth was monitored every hour by OD600 measurement. (B) Samples of WT FA1090 were collected at the times indicated. Whole cell lysates were separated by SDS-PAGE and probed with polyclonal rabbit anti-MlaA or anti-BamA (as a loading control) antisera. (C) Whole cell lysates of WT FA1090 were collected after 6 h culture in liquid medium containing desferal at concentrations ranging from 5–25 μM. Samples were probed with antisera against MlaA, MtrE, and TbpB. ΔmlaA cultured under standard conditions was included as a reference. (D) Densitometry analyses of MlaA using immunoblots from three independent desferal titration experiments shown as a representative blot in panel (C). (E) WT FA1090 and isogenic ΔmlaA were cultured in liquid medium containing either 10 μM (top panel) or 25 μM (bottom panel) desferal for 6 h. At each hour, samples were withdrawn and diluted for CFU/mL enumeration. Both graphs contain growth curves from cultures maintained under standard conditions (blue curves). Statistical significance was assessed by two-way ANOVA using Sidak’s multiple comparisons test. (F) WT FA1090 and isogenic knockout ΔmlaA were cultured in liquid medium under standard conditions until OD600 had at least doubled (~3 h). Cultures were standardized to an OD600 of 0.2 and dilutions were spotted onto GCB. Plates were prepared as normal (SGC); under iron deprivation (-Fe); supplemented with 7.5% normal human serum (+NHS); or supplemented with 1.2 mM NaNO2 and cultured anaerobically (-O2). Strains were maintained at 37 °C in 5% CO2 for approximately 22 h or at 37 °C anaerobically for 48 h and CFU were enumerated. (G) WT bacteria cultured as in (F), with the addition of a desferal titration from 0–25 μM under anaerobiosis, were collected from plates, separated by SDS-PAGE, and probed with polyclonal rabbit anti-MlaA antiserum or anti-BamA antiserum as a loading control. (H) Densitometry analyses of MlaA abundance under each host relevant condition presented in (G). Densitometry was performed twice on each of three independent experiments. (I) WT FA1090 and conditional knockout Δfur/Plac::fur were cultured in liquid medium in the absence (SGC) or presence of 25 μM desferal (-Fe). Fur expression was induced by the addition of 10, 50, or 100 μM IPTG. Samples were collected after 6 h of growth and probed with indicated antisera. (J) Densitometry analyses of MlaA abundance in immunoblots from three independent Fur induction experiments with and without iron starvation. (K) WT FA1090, isogenic knockout ΔmlaA, and ΔmlaA/Plac::mlaA were cultured aerobically in liquid medium until culture density had doubled (~3 h). Cultures were diluted to an OD600 of 0.05 in sterile PBS and diluted 1000-fold in EMEM. Suspensions were combined with an equal volume of EMEM, NHS, or heat-inactivated NHS and incubated for 1 h in 5% CO2 at 37 °C. Bacteria from each well were spotted onto GCB plates. CFUs were scored after 20–22 h incubation in 5% CO2 at 37 °C (p value between WT and ΔmlaA exposed to active NHS, 0.07). (L) Rapidly growing cultures of WT FA1090 and isogenic knockout ΔmlaA were diluted to 105 CFU/mL and cultured for 3 h in the presence of liquid medium (SGC), 0.01% acetic acid (vehicle), or 10 μM human defensin (HBD1). Bacteria were serially diluted and spotted onto GCB plates. CFUs were scored after 20–22 h incubation in 5% CO2 at 37 °C and survival was calculated relative to SGC. n≥3; mean ± SEM presented for all experiments; panels D, H, J, and L present values from each replicate; *p < 0.05; OD600, optical density at 600 nm; SGC, standard growth conditions; IPTG, isopropyl β-D-thiogalactopyranoside; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; CFU, colony forming unit; GCB, gonococcal base medium; GCBL, gonococcal base liquid medium; PBS, phosphate buffered saline; EMEM, Eagle’s minimal essential medium.