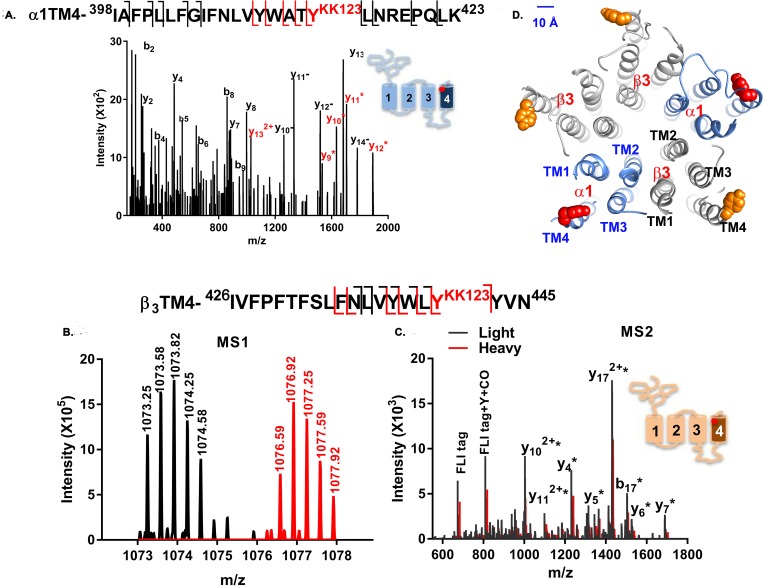
Fig 2. KK123 photolabels α1-TM4 and β3-TM4 peptides.
(A) A representative MS fragmentation spectrum of a KK123 photolabeled α1-TM4 peptide (m/z = 875.503, z = 4). The y9-y14 ions (red) contain the KK123 adduct. The site-defining ions y8 and y9 indicate that α1-Y415 (red) was photolabeled by KK123. Fragment ions y10− to y14− represent neutral loss of the KK123 adduct. (B). MS1 pair of light and heavy form of FLI-tag-KK123 photolabeled β3 TM4 peptide (m/z = 1,073.246 and m/z = 1,076.580, z = 3). (C) An overlay of light (black) and heavy (red) MS fragmentation spectra of FLI-tag-KK123 photolabeled β3 TM4 peptide. The KK123 adduct-containing b or y ions are labeled with “*”. Site-defining y4 and b17 ions identify β3-Y442 as the photolabeled residue. The photolabeled residues in α1- and β3-TM4 were identified in three replicate experiments. (D) KK123 photolabeled residues are shown in a homology model of the structure of an α1β3 GABAA receptor. In the α1 subunit, the labeled TM4 tyrosine (red) points toward TM1, whereas in the β3 subunit, the labeled tyrosine residue (orange) points toward TM3. The numerical data are included in S2 Data. GABAA, gamma amino-butyric acid Type A; MS, mass spectrometry; TM, transmembrane helix.