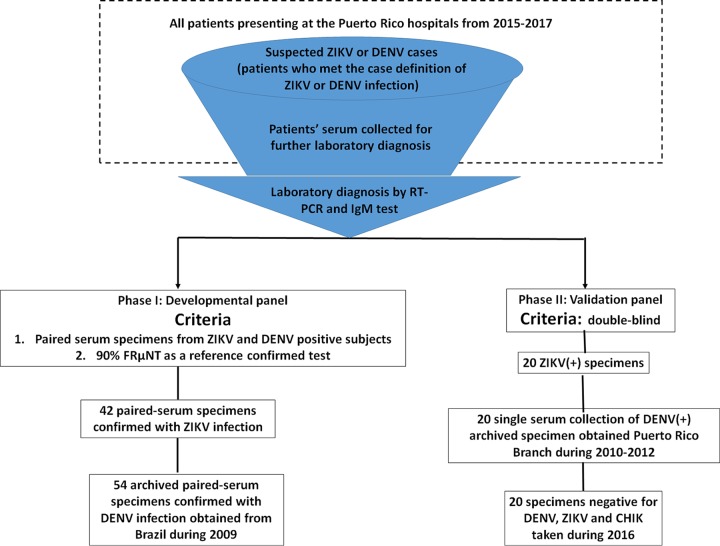
FIG 1.
Flow chart of subject recruitment for the serum panels and case classification during phases I and II. ZIKV-infected serum specimens were obtained from participants presenting at the Puerto Rico hospital from 2015 to 2017. Suspected cases of flavivirus infection were those with clinical symptoms matching the case reporting criteria defined by the CDC and were admitted to the hospital for further diagnosis. All patients were classified as having a ZIKV or a DENV infection based on the results of ZIKV- or DENV-specific RT-PCR analyses, respectively. Patients who tested negative by either ZIKV- or DENV-specific RT-PCR were further subjected to an IgM test for the sera collected during the convalescent phase. Only specimens that were ZIKV specific, were RT-PCR positive in the acute phase, and had seroconversion of IgM in the convalescent phase were included here. Those without confirmations by RT-PCR or IgM laboratory results were excluded. The criteria of all ZIKV- and DENV-confirmed specimens used in phase I included that the paired sera were collected during the acute and convalescent phases of illness and that the disease status was further determined by an FRµNT90 on ZIKV and DENV-2 in order to properly establish the cutoff value of our assay. The only criterion for the serum panel used in phase II is that it was double blind. All DENV-positive specimens were collected during 2010 to 2012 during the time the paired specimen from the same patient was rare. The negative specimens were obtained during 2016, and they are all negative by RT-PCR assay for DENV, ZIKV, and chikungunya virus (CHIK) in the acute phase and negative for IgM in convalescent-phase samples.