Abstract
The aim of the present study was to investigate the potential prognostic value of members of the heat shock protein (HSP)90 family in non-small cell lung cancer (NSCLC) patients. The mRNA expression profiles of 1,926 NSCLC patients, which was available from the Kaplan-Meier plotter database, were included in the study. High expression of HSP90AA1 mRNA was significantly associated with a poorer rate of overall survival (OS) for all NSCLC patients [hazard ratio (HR), 1.21; 95% confidence interval (CI): 1.06–1.37; P=0.004], as well as for patients with adenocarcinoma (ADE; HR, 1.3; 95% CI: 1.02–1.65; P=0.034), but no significant correlation was identified for squamous cell carcinoma (SCC) patients (HR, 1.08; 95% CI: 0.85–1.38; P=0.51). High expression of HSP90AB1 and HSP90B1 mRNA was significantly associated with poorer rates of OS in lung SCC and ADE patients combined, as well as in lung ADE patients alone. By contrast, high expression of tumor necrosis factor receptor-associated protein 1 (TRAP1) mRNA was significantly associated with improved OS rates in all NSCLC patients combined (HR, 0.88; 95% CI: 0.77–0.99; P=0.041), as well as ADE patients. In stratified survival analysis, a high expression of HSP90AA1, HSP90AB1 and HSP90B1 predicted poor prognosis in stage I NSLCC patients, suggesting that these genes may serve as stage-independent prognostic indicators. As an elevated expression of HSP90AA1, HSP90AB1, HSP90B1 and TRAP1 was associated with poorer OS outcomes in patients with NSCLC, these HSP90 members may be potential prognostic biomarkers and drug targets for the treatment of NSCLC.
Keywords: non-small cell lung cancer, heat shock protein 90, Kaplan-Meier plotter, prognosis, database
Introduction
Lung cancer was reported to be the most common cancer type in males in 2016, and is associated with a poor prognosis (1). Non-small cell lung cancer (NSCLC) accounts for 80–85% of all cases of lung cancer, with squamous cell carcinoma (SCC) and adenocarcinoma (ADE) being the most common histological subtypes (2,3). Despite advances in the early detection and therapeutic techniques, the overall 5-year survival rate is low. Consequently, further investigations into the mechanisms of initiation and progression, and the development of prognostic biomarkers and drug targets are required in order to improve the prognosis for NSCLC and allow for personalized therapy.
Heat shock protein 90 (HSP90) is one of the most widespread heat-associated proteins. HSP90 forms flexible homodimers and its basic structure comprises three parts: The N-terminal domain, the middle domain and the C-terminal domain. HSP90 is overexpressed in various cancer types, including pancreatic, ovarian, breast, lung and endometrial cancer, as well as oropharyngeal SCC and multiple myeloma (4–7). High expression of HSP90 was indicated to be a marker of poor prognosis in lung cancer, esophageal cancer, bladder cancer, melanoma and leukemia (8–11). Combination therapy with HSP90 inhibitors and conventional photon radiation delays tumor growth more effectively than radiotherapy alone (12). The HSP90 inhibitor NVP-AUY922 induces cell death in the lung and is currently undergoing clinical trials in lung cancer patients (13). However, the prognostic value of HSP90 in NSCLC has remained to be determined.
In the present study, the Kaplan-Meier (KM) plotter database (http://kmplot.com/analysis/index.php?p=service&cancer=lung) was employed to assess the correlation between HSP90 mRNA expression and overall survival (OS). At present, the KM plotter database comprises gene expression information and clinical outcome parameters of various types of cancer (14–18). The KM plotter database may be used to analyze individual genes that may correlate with the OS of NSCLC patients. To date, the KM plotter database has been used to identify and validate numerous genes involved in NSCLC (15–18). In the present study, this database was used to evaluate the prognostic value of individual HSP90 protein members in patients with NSCLC.
Materials and methods
The information on the NSCLC patients contained in the KM plotter database was extracted from the Gene Expression Omnibus (GEO), the Cancer Biomedical Informatics Grid and The Cancer Genome Atlas database. The following NSCLC datasets were obtained from the GEO database: GSE14814, GSE19188, GSE29013, GSE30219, GSE31210, GSE3141, GSE31908, GSE37745, GSE43580, GSE4573, GSE50081 and GSE8894 (http://kmplot.com/analysis/index.php?p=service&cancer=lung) (19). The KM plotter database was used to analyze the association of the mRNA expression of individual HSP90 members with relapse-free survival. WinStat 2013 software was used as an analysis tool. Data regarding age, sex, smoking history, histology, stage, success of surgery, radiotherapy and applied chemotherapy were recorded for all patients. In general, five HSP90 subfamily members [HSP90AA1, HSP90AA2, HSP90AB1, HSP90B1 and tumor necrosis factor receptor-associated protein 1 (TRAP1)] were included in the KM plotter analysis to acquire KM survival plots, in which the number of patients at risk for certain time-points is compared between subgroups with different gene expression status. The hazard ratio (HR) and 95% confidence intervals (CI) and log-rank P-values were determined. A total of 1,926 lung cancer patients were included in the study. When the P-value was <0.05, the difference was regarded as statistically significant.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/) was used to perform gene ontology (GO) functional annotation analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of various HSP90 members. Gene-gene interaction networks were generated with GeneMANIA (http://genemania.org/). Protein-protein interaction networks were constructed with the Search Tool for the Retrieval if Interacting Genes and Proteins (STRING; http://string-db.org/).
Results
Prognostic value of HSP90 members in NSCLC
The HSP90 family comprises five sub-members (HSP90AA1, HSP90AA2, HSP90AB1, HSP90B1 and TRAP1). Of these, only HSP90AA2 was not included in the KM plot database, possibly due to its low levels of expression.
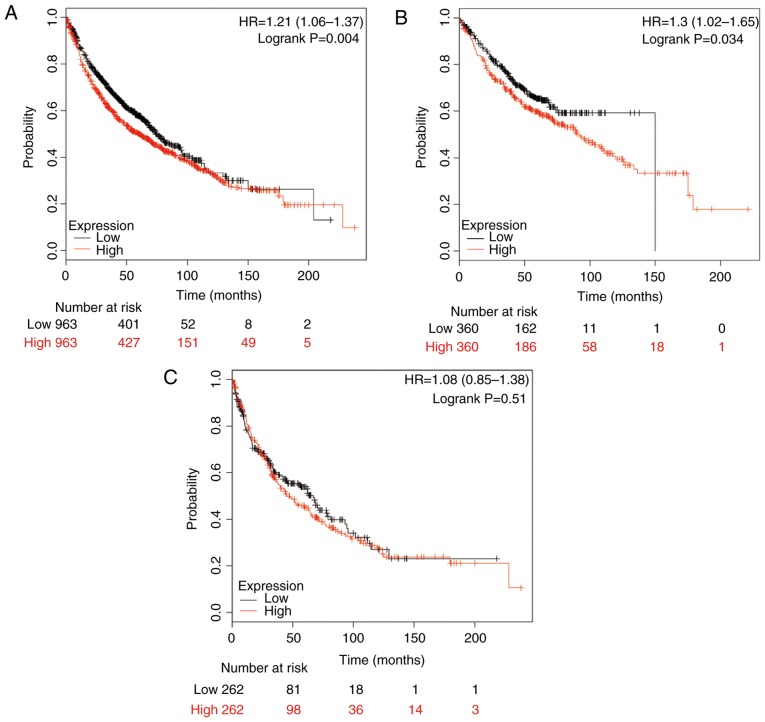
First, the prognostic value of the mRNA expression of HSP90AA1 was evaluated in the dataset. The valid gene Affymetrix ID was 211969_at (HSP90AA1). Survival curves were generated to draw preliminary conclusions regarding the influence of HSP90AA1 on the prognosis of patients with NSCLC (n=1,926; Fig. 1A), ADE (n=720; Fig. 1B) and SCC (n=524; Fig. 1C). A high expression of HSP90AA1 mRNA was significantly associated with a worse OS for all NSCLC cases (HR, 1.21, 95% CI: 1.06–1.37; P=0.004; Fig. 1A). High expression of HSP90AA1 mRNA was also significantly associated with poor rates of OS in ADE patients (HR, 1.30; 95% CI: 1.02–1.65; P=0.034; Fig. 1B), while the association in SCC patients was not significant (HR, 1.08; 95% CI, 0.85–1.38; P=0.51; Fig. 1C).
Figure 1.
Kaplan-Meier analysis of the prognostic value of HSP90AA1 mRNA expression in the dataset [Affymetrix ID, 211969_at (HSP90AA1)]. Survival curves were plotted for (A) all non-small cell lung cancer patients (n=1,926), (B) those with adenocarcinoma (n=720) and (C) those with squamous cell carcinoma (n=524). Cut-off value, median expression level. HSP, heat shock protein; HR, hazard ratio (with 95% confidence interval).
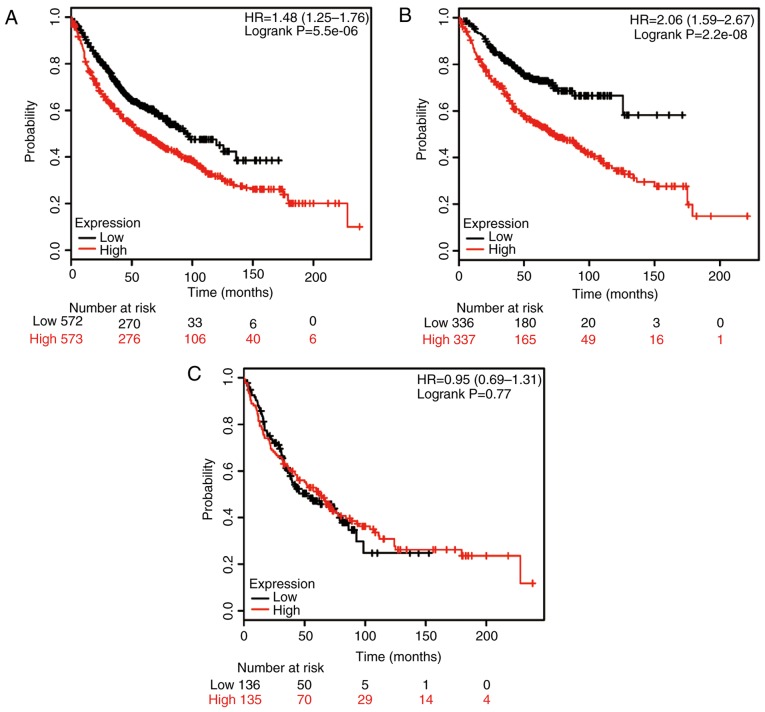
Next, the potential prognostic role of HSP90AB1 mRNA expression was evaluated using the database. The valid gene Affymetrix ID was 1557910_at (HSP90AB1). High expression of HSP90AB1 mRNA was significantly associated with poor rates of OS for all NSCLC patients (HR, 1.48; 95% CI: 1.25–1.76; P=5.5×10−6; Fig. 2A). High expression of HSP90AB1 mRNA was also associated with poor rates of OS in ADE patients (HR, 2.06; 95% CI: 1.59–2,67; P=2.2×10−8; Fig. 2B), but the correlation with OS rates in SCC patients was not significant (HR, 0.95; 95% CI: 0.69–1.31; P=0.77; Fig. 2C).
Figure 2.
Kaplan-Meier analysis of the prognostic value of HSP90AB1 mRNA expression in the dataset [Affymetrix ID, 1557910_at (HSP90AB1)]. Survival curves were plotted for (A) all non-small cell lung cancer patients (n=1,926), (B) those with adenocarcinoma (n=720) and (C) those with squamous cell carcinoma (n=524). Cut-off value, median expression level. HSP, heat shock protein; HR, hazard ratio (with 95% confidence interval).
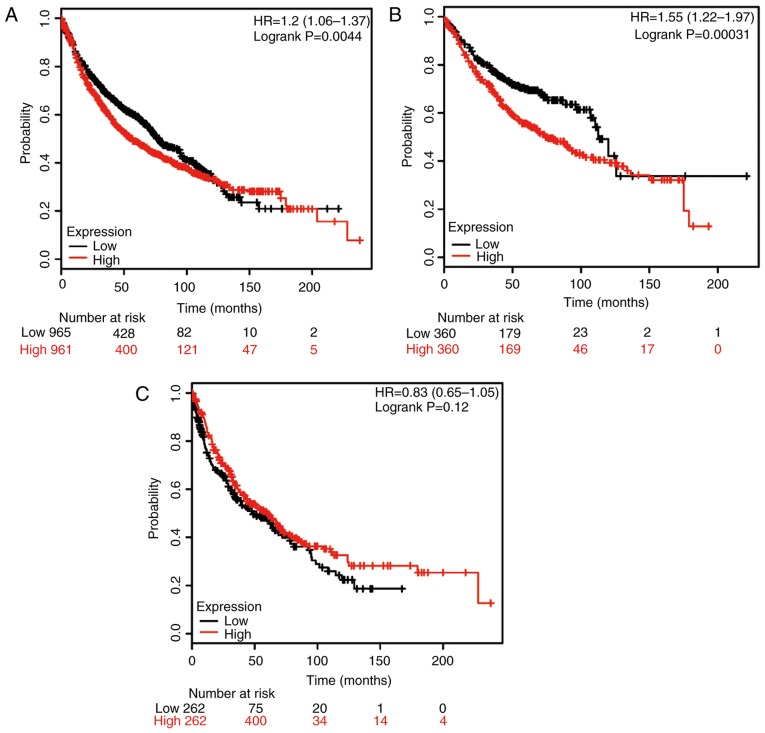
Furthermore, the potential prognostic value of HSP90B1 mRNA expression was evaluated using the database (Fig. 3). The valid gene Affymetrix ID was 216449_x_at (HSP90B1). High expression of HSP90B1 mRNA was significantly associated with poor rates of OS for all NSCLC patients (HR, 1.20; 95% CI: 1.06–1.37; P=0.0044; Fig. 3A), as well as for ADE patients (HR, 1.55; 95% CI: 1.22–1.97; P=0.00031; Fig. 3B); however, no significant association was obtained for SCC patients (HR, 0.83; 95% CI: 0.65–1.05; P=0.12; Fig. 3C).
Figure 3.
Kaplan-Meier analysis of the prognostic value of HSP90B1 mRNA expression in the dataset [Affymetrix ID, 216449_x_at (HSP90B1)]. Survival curves were plotted for (A) all non-small cell lung cancer patients (n=1,926), (B) those with adenocarcinoma (n=720) and (C) those with squamous cell carcinoma (n=524). Cut-off value, median expression level. HSP, heat shock protein; HR, hazard ratio (with 95% confidence interval).
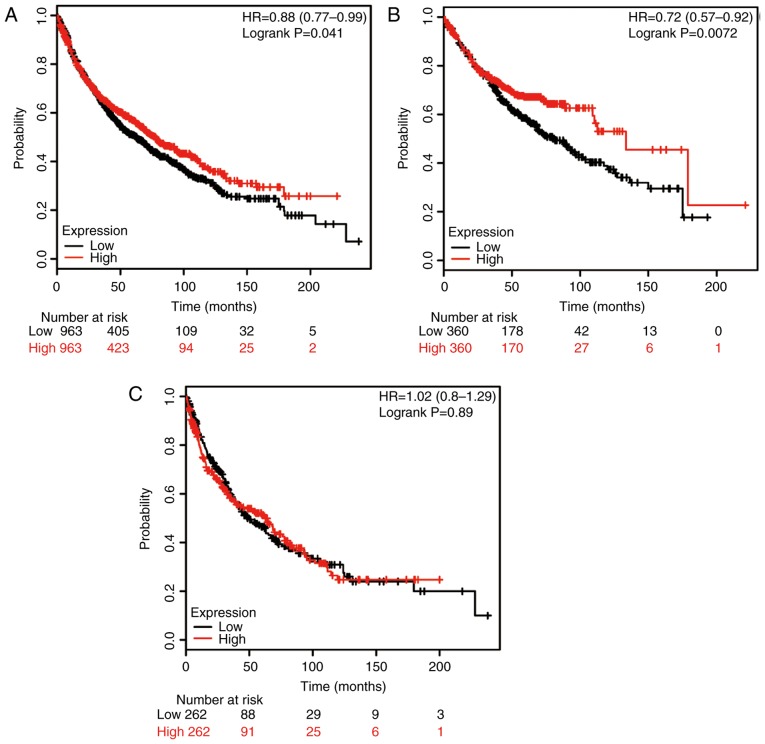
Finally, the potential prognostic role of TRAP1 mRNA expression was evaluated using the database. The valid gene Affymetrix ID was 201391_at (TRAP1). High expression of TRAP1 mRNA was significantly associated with higher OS rates for all patients (HR, 0.88; 95% CI: 0.77–0.99; P=0.041; Fig. 4A), as well as for ADE patients (HR, 0.72; 95% CI: 0.57–0.92; P=0.0072; Fig. 4B); however, no significant association was identified for SCC patients (HR, 1.02; 95% CI: 0.80–1.29; P=0.89; Fig. 4C).
Figure 4.
Kaplan-Meier analysis of the prognostic value of TRAP1 mRNA expression in the dataset [Affymetrix ID, 201391_at (TRAP1)]. Survival curves were plotted for (A) all non-small cell lung cancer patients (n=1,045), (B) those with adenocarcinoma (n=673) and (C) those with squamous cell carcinoma (n=217). Cut-off value, median expression level. TRAP1, tumor necrosis factor receptor-associated protein 1; HR, hazard ratio (with 95% confidence interval).
Next, to assess whether the prognostic value of the mRNA expression status of individual HSP90 members depends on other clinicopathological factors, the influence of high vs. low expression on OS was determined for NSCLC patients stratified based on the following parameters: The patients' smoking history, clinical stage of the disease and chemotherapy status. As presented in Table I, high expression of HSP90AA1, HSP90B1 and TRAP1 mRNA had no significant prognostic value in patients with or without a history of smoking. However, a high expression of HSP90AB1 was identified in patients with a smoking history and HSP90B1 in those that did not smoke. Table II reveals that there was only a significant prognostic value for HSP90AA1 and HSP90AB1 in stage I patients, and for HSP90B1 in stage I and II patients, but not in patients at stage III. No significant prognostic values were observed in all stages of TRAP1 in NSCLC. No significant association with OS was identified for any of the HSP90 members in patients that did or did not undergo chemotherapy (Table III).
Table I.
Influence of HSP90 mRNA expression on the prognosis of non-small cell lung cancer patients with a history of smoking (n=820) and those who never smoked (n=205).
| Gene/smoking history | HR | 95% CI | P-value |
|---|---|---|---|
| HSP90AA1 | |||
| Never smoked | 0.81 | 0.46–1,42 | 0.46 |
| Smoked | 1.14 | 0.93–1.40 | 0.22 |
| HSP90AB1 | |||
| Never smoked | 1.89 | 0.84–4.33 | 0.12 |
| Smoked | 1.59 | 1.05–2.42 | 0.029 |
| HSP90B1 | |||
| Never smoked | 3.51 | 1.86–6.62 | 3.40×10−5 |
| Smoked | 1.11 | 0.90–1.37 | 0.31 |
| TRAP1 | |||
| Never smoked | 0.73 | 0.42–1.28 | 0.27 |
| Smoked | 0.92 | 0.75–1.13 | 0.43 |
TRAP1, tumor necrosis factor receptor-associated protein 1; HSP, heat shock protein; HR, hazard ratio; CI, confidence interval
Table II.
Influence of HSP90 mRNA expression on the prognosis of non-small cell lung cancer patients with the clinical stage of I (n=577), II (n=244) and III (n=70).
| Gene/clinical stage | HR | 95% CI | P-value |
|---|---|---|---|
| HSP90AA1 | |||
| I | 1.56 | 1.29–1.88 | 2.70×10−6 |
| II | 1.18 | 0.82–1.71 | 0.38 |
| III | 1.02 | 0.58–1.79 | 0.94 |
| HSP90AB1 | |||
| I | 3.28 | 2.29–4.7 | 7.40×10−12 |
| II | 1.66 | 1.04–2.66 | 0.033 |
| III | 1.34 | 0.64–2.79 | 0.44 |
| HSP90B1 | |||
| I | 1.86 | 1.40–2.47 | 1.5×10−5 |
| II | 1.1 | 0.76–1.59 | 0.61 |
| III | 0.92 | 0.54–1.59 | 0.78 |
| TRAP1 | |||
| I | 0.83 | 0.63–1.09 | 0.17 |
| II | 1.14 | 0.79–1.65 | 0.48 |
| III | 0.68 | 0.39–1.17 | 0.16 |
TRAP1, tumor necrosis factor receptor-associated protein 1; HSP, heat shock protein; HR, hazard ratio; CI, confidence interval
Table III.
Influence of HSP90 mRNA expression on the prognosis of non-small cell lung cancer patients with chemotherapy (n=176) and without chemotherapy (n=310).
| Genes/chemotherapy | HR | 95% CI | P-value |
|---|---|---|---|
| HSP90AA1 | |||
| No | 1.17 | 0.84–1.64 | 0.36 |
| Yes | 0.91 | 0.60–1.39 | 0.67 |
| HSP90AB1 | |||
| No | 0.79 | 0.16–3.98 | 0.78 |
| Yes | 0.73 | 0.23–2.33 | 0.59 |
| HSP90B1 | |||
| No | 0.86 | 0.62–1.20 | 0.38 |
| Yes | 0.87 | 0.58–1.32 | 0.52 |
| TRAP1 | |||
| No | 1.16 | 0.83–1.62 | 0.39 |
| Yes | 1.27 | 0.84–1.89 | 0.25 |
TRAP1, tumor necrosis factor receptor-associated protein 1; HSP, heat shock protein; HR, hazard ratio; CI, confidence interval
GO functional annotation analysis and KEGG pathway analysis of HSP90 members
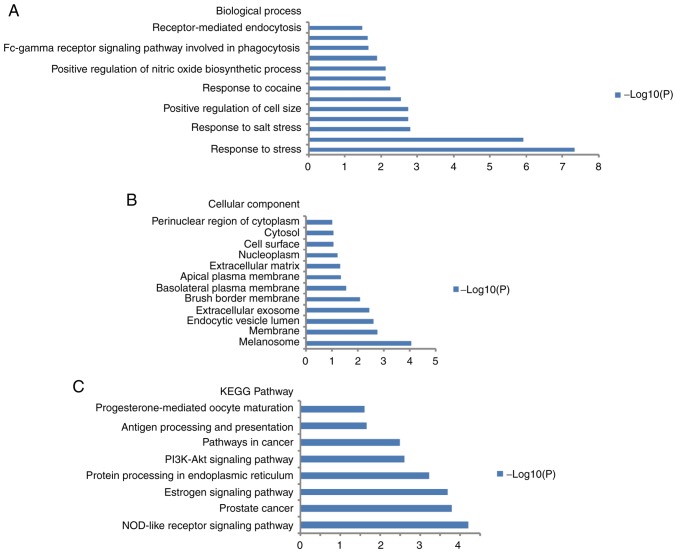
DAVID was used to investigate the biological functions of the HSP90 members (HSP90AA1, HSP90B1, HSP90AB1 and TRAP1). GO enrichment analysis in the categories cellular component and biological process was performed (Fig. 5A and B). The results revealed that cell size was positively regulated and the response to stress was enriched. Enrichment of certain genes interacting with HSP90 family members in certain KEGG pathways was also identified (Fig. 5C) and included the phosphatidylinositol 3′kinase (PI3K)-Akt signaling pathway in cancer.
Figure 5.
Analysis of GO terms and KEGG performed using the Database for the Annotation, Visualization and Integrated Discovery. (A) GO terms in the category biological processes. (B) GO terms in the category cellular component. (C) KEGG pathway analysis. GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PI3K, phosphoinositide-3 kinase.
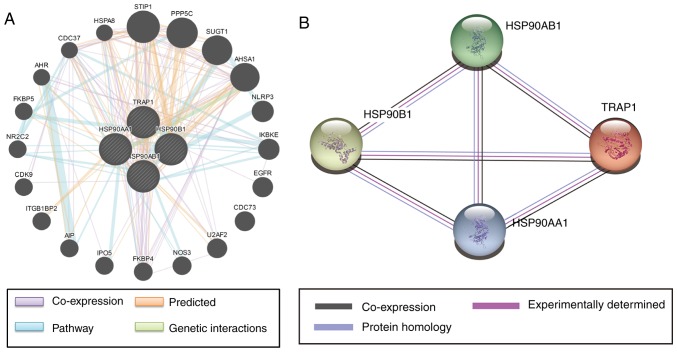
Gene-gene interaction networks were constructed with GeneMANIA (Fig. 6A) and protein-protein interaction networks were constructed using STRING (Fig. 6B). It was indicated that the HSP90 family has complex interactions, and that sub-members of the HSP90 family are co-expressed with each other.
Figure 6.
(A) Gene-gene interaction networks among selected genes constructed by GeneMANIA. (B) Protein-protein interaction networks among selected proteins constructed with the Search Tool for the Retrieval if Interacting Genes and Proteins. The size of the circles indicates the degree of interaction. HSP, heat shock protein; TRAP1, tumor necrosis factor receptor-associated protein 1; STIP1, stress induced phosphoprotein 1; PPP5C, protein phosphatase 5 catalytic; SUGT1, suppressor of G2 allele of sKP1; AHSA1, activator of HSP90 ATPase activity 1; NLRP3, NLR family pyrin domain containing 3; IKBKE, inhibitor of nuclear factor kappa B kinase subunit epsilon; EGFR, epidermal growth factor receptor; CDC73, cell division cycle 73; U2AF2, U2 small nuclear RNA auxiliary factor 2; NOS3, nitric oxide synthase 3; FKBP4, FK506 binding protein 4; IPO5, importin 5; AIP, aryl hydrocarbon receptor interacting protein; ITGB1BP2, integrin subunit beta 1 binding protein 2; CDK9, cyclin dependent kinase 9; NR2C2, nuclear receptor subfamily 2 group C member 2; FKBP5, FK506 binding protein 5; AHR, aryl hydrocarbon receptor; CDC37, cell division cycle 37; HSPA8, heat shock protein family A member 8.
Discussion
NSCLC is a highly malignant type of cancer with a poor 5-year survival rate (3,20). HSP90 has been reported to have a role in numerous types of cancer, and overexpression of this protein has been proposed to enhance carcinogenesis and affect the prognosis of patients (21–25). HSP90α, the protein encoded by HSP90AA1, is considered to have an important regulatory role in tumor invasion and migration (26,27). The present study indicated an influence of the HSP90 expression status on the OS rate of NSCLC patients. An association between HSP90 and the clinical features of NSCLC was also observed.
HSP90α is secreted extracellularly and has an important role in wound healing and inflammation. These two processes are frequently hijacked by cancer, leading to malignant cell motility, metastasis and extravasion (28). HSP90α modulates growth and lung metastasis by affecting the proliferation, migration and invasion of isolated primary carcinoma cells (29). One study indicated that the level of HSP90α in the plasma of patients with the advanced stages of malignant tumors was relatively high (30). The expression of HSP90α has been reported to correlate with the degree of malignancy of esophageal SCC and the prognosis of affected patients (8). Furthermore, the present study suggested that a high expression of HSP90AA1 mRNA was associated with a poor prognosis in ADE patients, but not in SCC patients. Overall, it may be concluded that high expression of HSP90α mRNA correlates with poorer rates of OS in all NSCLC patients combined.
In previous study, the expression rate of HSP90AB1 in lung ADE tissues was determined to be obviously higher than that in lung SCC tissues, and its overexpression was associated with poor prognosis for lung ADE patients (31). In the present study, high expression of HSP90AB1 mRNA was significantly associated with a poorer OS rate in ADE patients but not in SCC patients.
HSP90B1 is essential for mediating the contribution of tumor-associated macrophages to inflammatory colon tumorigenesis in mice (32). Overexpression of HSP90β leads to significantly increased proliferation, migration, invasion and tube formation of human endothelial cells. HSP90β was reported to promote endothelial cell-dependent tumor angiogenesis by increasing vascular endothelial growth factor receptor expression (33). Serum HSP90B1 levels were reported to be significantly elevated in dogs with mammary tumors compared with those in healthy controls (34). Another study demonstrated that HSP90B1 is an oncogenic chaperone in hepatocyte carcinogenesis (35). Overexpression of HSP90B1 may be associated with the poor survival rate of hepatocellular carcinoma patients (36). In patients with early-stage liver cancer, a considerable increase in HSP90B1 is associated with tumor metastasis and recurrence (37). Analysis of survival rates demonstrated that HSP expression was an independent unfavorable prognostic factor for gall bladder cancer (38). Furthermore, HSP90B1 overexpression was indicated to be an accurate predictive factor for shorter OS and disease-free survival in NSCLC patients (39).
High TRAP1 expression has previously been reported to be an adverse prognostic factor for patients with NSCLC (40,41). Conversely, the present results indicated that TRAP1 mRNA expression is correlated with an improved prognosis in all NSCLC patients combined, as well as ADE patients alone. The significance of TRAP1 in NSCLC therefore requires further investigation.
Nicotine, a major addictive component of tobacco smoke, has been reported to induce cell cycle progression, angiogenesis and metastasis during cancer (42,43). However, the potential role of nicotine in HSP90 activation in NSCLC patients has not been previously addressed, to the best of our knowledge. Nicotine is known to induce apoptosis in human cells, possibly through increased HSP90α expression (44). In the present study, a high expression of HSP90AB1 mRNA was identified to be a significant prognostic factor in NSCLC patients with a smoking history, but not in those who never smoked. However, high expression of HSP90B1 mRNA was identified to be a significant prognostic factor in NSCLC patients who never smoked, but not in those with a smoking history. Further research is required to confirm these results and identify the possible underlying mechanisms.
Previous studies have proposed that HSP90 may be a crucial drug target for the treatment of NSCLC. For instance, inhibition of the mitochondrial HSP90 network may be an effective treatment for highly refractory tumors (45), and topical application of the HSP90 inhibitor 17AAG is effective in preventing epidermal hyperplasia and SCC (46).
The current study has some limitations that need clarification. A stratified analysis for all NSCLC patients combined was performed, but included patients with SCC, which demonstrated no significance. Therefore, a stratified survival analysis for each type of NSCLC should be performed separately with a large sample size in the future.
In conclusion, the results of the present study suggest that HSP90AA1, HSP90AB1 and HSP90B1 may be potential biomarkers for the prognosis of NSCLC. However, the present results were obtained using bioinformatics methods and further research is required to explore the underlying molecular mechanisms in the future.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 81460460, 81360405 and 81760542), the Foundation of Sharing Project Based on Tumor Precise Radiotherapy (grant no. ZY18076006), the China Postdoctoral Science Foundation (grant no. 2016M602918XB) and the Research Foundation of the Science and Technology Department of Guangxi Province, China (grant nos. 2016GXNSFAA380252 and 2014GXNSFBA118114).
Authors' contributions
RW designed the study and performed bioinformatics analysis. KL analyzed the data and wrote the manuscript. MK, WQ and JL analyzed the data. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethical approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/S1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 4.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–1536. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 5.Kolosenko I, Grander D, Tamm KP. IL-6 activated JAK/STAT3 pathway and sensitivity to Hsp90 inhibitors in multiple myeloma. Curr Med Chem. 2014;21:3042–3047. doi: 10.2174/0929867321666140414100831. [DOI] [PubMed] [Google Scholar]
- 6.Patel K, Wen J, Magliocca K, Muller S, Liu Y, Chen ZG, Saba N, Diaz R. Heat shock protein 90 (HSP90) is overexpressed in p16-negative oropharyngeal squamous cell carcinoma, and its inhibition in vitro potentiates the effects of chemoradiation. Cancer Chemother Pharmacol. 2014;74:1015–1022. doi: 10.1007/s00280-014-2584-8. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Liu X, Lou J, Han X, Zhang L, Wang Q, Li B, Dong M, Zhang Y. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res. 2014;20:6016–6022. doi: 10.1158/1078-0432.CCR-14-0174. [DOI] [PubMed] [Google Scholar]
- 8.Huang T, Chen S, Han H, Li H, Huang Z, Zhang J, Yin Q, Wang X, Ma X, Dai P, et al. Expression of Hsp90alpha and cyclin B1 were related to prognosis of esophageal squamous cell carcinoma and keratin pearl formation. Int J Clin Exp Pathol. 2014;7:1544–1552. [PMC free article] [PubMed] [Google Scholar]
- 9.Tian WL, He F, Fu X, Lin JT, Tang P, Huang YM, Guo R, Sun L. High expression of heat shock protein 90 alpha and its significance in human acute leukemia cells. Gene. 2014;542:122–128. doi: 10.1016/j.gene.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy MM, Pick E, Kluger Y, Gould-Rothberg B, Lazova R, Camp RL, Rimm DL, Kluger HM. HSP90 as a marker of progression in melanoma. Ann Oncol. 2008;19:590–594. doi: 10.1093/annonc/mdm545. [DOI] [PubMed] [Google Scholar]
- 11.Záčková M, Moučková D, Lopotová T, Ondračková Z, Klamová H, Moravcová J. Hsp90-a potential prognostic marker in CML. Blood Cells Mol Dis. 2013;50:184–189. doi: 10.1016/j.bcmd.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa H, Fujisawa H, Masaoka A, Noguchi M, Hirayama R, Takahashi M, Fujimori A, Okayasu R. The combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation provides effective tumor control in human lung cancer cells. Cancer Med. 2015;4:426–436. doi: 10.1002/cam4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson ML, Yu HA, Hart EM, Weitner BB, Rademaker AW, Patel JD, Kris MG, Riely GJ. Phase I/II study of HSP90 inhibitor AUY922 and erlotinib for EGFR-mutant lung cancer with acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol. 2015;33:1666–1673. doi: 10.1200/JCO.2014.59.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 15.Huang ZC, Li H, Sun ZQ, Zheng J, Zhao RK, Chen J, Sun SG, Wu CJ. Distinct prognostic roles of HSPB1 expression in non-small cell lung cancer. Neoplasma. 2018;65:161–166. doi: 10.4149/neo_2018_102. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt LH, Brand C, Stucke-Ring J, Schliemann C, Kessler T, Harrach S, Mohr M, Gorlich D, Marra A, Hillejan L, et al. Potential therapeutic impact of CD13 expression in non-small cell lung cancer. PLoS One. 2017;12:e0177146. doi: 10.1371/journal.pone.0186280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie S, Shen C, Tan M, Li M, Song X, Wang C. Systematic analysis of gene expression alterations and clinical outcomes of adenylate cyclase-associated protein in cancer. Oncotarget. 2017;8:27216–27239. doi: 10.18632/oncotarget.16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou GX, Liu P, Yang J, Wen S. Mining expression and prognosis of topoisomerase isoforms in non-small-cell lung cancer by using Oncomine and Kaplan-Meier plotter. PLoS One. 2017;12:e0174515. doi: 10.1371/journal.pone.0174515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Győrffy B, Surowiak P, Budczies J, Lanczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8:e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukas RV, Gondi V, Kamson DO, Kumthekar P, Salgia R. State-of-the-art considerations in small cell lung cancer brain metastases. Oncotarget. 2017;8:71223–71233. doi: 10.18632/oncotarget.19333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calderwood SK, Neckers L. Hsp90 in cancer: Transcriptional roles in the nucleus. Adv Cancer Res. 2016;129:89–106. doi: 10.1016/bs.acr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Wang J, Huang Z, Wei P, Liu Y, Hao J, Zhao L, Zhang F, Tu Y, Wei T. Aberrantly upregulated TRAP1 is required for tumorigenesis of breast cancer. Oncotarget. 2015;6:44495–44508. doi: 10.18632/oncotarget.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmieri C, Mancini M, Benazzi C, Della Salda L. Heat shock protein 90 is associated with hyperplasia and neoplastic transformation of canine prostatic epithelial cells. J Comp Pathol. 2014;150:393–398. doi: 10.1016/j.jcpa.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Badowska-Kozakiewicz AM, Malicka E. Immunohistochemical evaluation of expression of heat shock proteins HSP70 and HSP90 in mammary gland neoplasms in bitches. Pol J Vet Sci. 2012;15:209–214. doi: 10.2478/v10181-011-0135-3. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Kang KW, Kim JE, Hwang SW, Park JH, Kim SH, Ji JH, Kim TG, Nam HY, Roh MS, et al. Differential expression of heat shock protein 90 isoforms in small cell lung cancer. Int J Clin Exp Pathol. 2015;8:9487–9493. [PMC free article] [PubMed] [Google Scholar]
- 26.Wong DS, Jay DG. Emerging roles of extracellular Hsp90 in cancer. Adv Cancer Res. 2016;129:141–163. doi: 10.1016/bs.acr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90α assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One. 2011;6:e18848. doi: 10.1371/journal.pone.0018848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 29.Vartholomaiou E, Madon-Simon M, Hagmann S, Muhlebach G, Wurst W, Floss T, Picard D. Cytosolic Hsp90α and its mitochondrial isoform Trap1 are differentially required in a breast cancer model. Oncotarget. 2017;8:17428–17442. doi: 10.18632/oncotarget.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc Natl Acad Sci USA. 2009;106:21288–21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Feng L, Li P, Han N, Gao Y, Xiao T. Hsp90AB1 protein is overexpressed in non-small cell lung cancer tissues and associated with poor prognosis in lung adenocarcinoma patients. Zhongguo Fei Ai Za Zhi. 2016;19:64–69. doi: 10.3779/j.issn.1009-3419.2016.02.02. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales C, Rachidi S, Hong F, Sun S, Ouyang X, Wallace C, Zhang Y, Garret-Mayer E, Wu J, Liu B, Li Z. Immune chaperone gp96 drives the contributions of macrophages to inflammatory colon tumorigenesis. Cancer Res. 2014;74:446–459. doi: 10.1158/0008-5472.CAN-13-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng J, Liu Y, Han J, Tan Q, Chen S, Qiao K, Zhou H, Sun T, Yang C. Hsp90β promoted endothelial cell-dependent tumor angiogenesis in hepatocellular carcinoma. Mol Cancer. 2017;16:72. doi: 10.1186/s12943-017-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunil Kumar BV, Bhardwaj R, Mahajan K, Kashyap N, Kumar A, Verma R. The overexpression of Hsp90B1 is associated with tumorigenesis of canine mammary glands. Mol Cell Biochem. 2018;440:23–31. doi: 10.1007/s11010-017-3152-4. [DOI] [PubMed] [Google Scholar]
- 35.Rachidi S, Sun S, Wu BX, Jones E, Drake RR, Ogretmen B, Cowart LA, Clarke CJ, Hannun YA, Chiosis G, et al. Endoplasmic reticulum heat shock protein gp96 maintains liver homeostasis and promotes hepatocellular carcinogenesis. J Hepatol. 2015;62:879–888. doi: 10.1016/j.jhep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Zhuang L, Szatmary P, Wen L, Sun H, Lu Y, Xu Q, Chen X. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci. 2015;12:256–263. doi: 10.7150/ijms.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou J, Li X, Li C, Sun L, Zhao Y, Zhao J, Meng S. Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol Oncol. 2015;9:1312–1323. doi: 10.1016/j.molonc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Chen C, Ma C, Sun S, Zhang J, Sun Y. Expression of heat-shock protein gp96 in gallbladder cancer and its prognostic clinical significance. Int J Clin Exp Pathol. 2015;8:1946–1953. [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Chen Z, Zhang G, Xi Y, Sun R, Wang X, Wang W, Chai F, Li X. HSP90B1 overexpression predicts poor prognosis in NSCLC patients. Tumour Biol. 2016;37:14321–14328. doi: 10.1007/s13277-016-5304-7. [DOI] [PubMed] [Google Scholar]
- 40.Agorreta J, Hu J, Liu D, Delia D, Turley H, Ferguson DJ, Iborra F, Pajares MJ, Larrayoz M, Zudaire I, et al. TRAP1 regulates proliferation, mitochondrial function, and has prognostic significance in NSCLC. Mol Cancer Res. 2014;12:660–669. doi: 10.1158/1541-7786.MCR-13-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sisinni L, Maddalena F, Condelli V, Pannone G, Simeon V, Li Bergolis V, Lopes E, Piscazzi A, Matassa DS, Mazzoccoli C, et al. TRAP1 controls cell cycle G2-M transition through the regulation of CDK1 and MAD2 expression/ubiquitination. J Pathol. 2017;243:123–134. doi: 10.1002/path.4936. [DOI] [PubMed] [Google Scholar]
- 42.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardinale A, Nastrucci C, Cesario A, Russo P. Nicotine: Specific role in angiogenesis, proliferation and apoptosis. Crit Rev Toxicol. 2012;42:68–89. doi: 10.3109/10408444.2011.623150. [DOI] [PubMed] [Google Scholar]
- 44.Wu YP, Kita K, Suzuki N. Involvement of human heat shock protein 90 alpha in nicotine-induced apoptosis. Int J Cancer. 2002;100:37–42. doi: 10.1002/ijc.10449. [DOI] [PubMed] [Google Scholar]
- 45.Siegelin MD. Inhibition of the mitochondrial Hsp90 chaperone network: A novel, efficient treatment strategy for cancer? Cancer Lett. 2013;333:133–146. doi: 10.1016/j.canlet.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Singh A, Singh A, Sand JM, Bauer SJ, Hafeez BB, Meske L, Verma AK. Topically applied Hsp90 inhibitor 17AAG inhibits UVR-induced cutaneous squamous cell carcinomas. J Invest Dermatol. 2015;135:1098–1107. doi: 10.1038/jid.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.