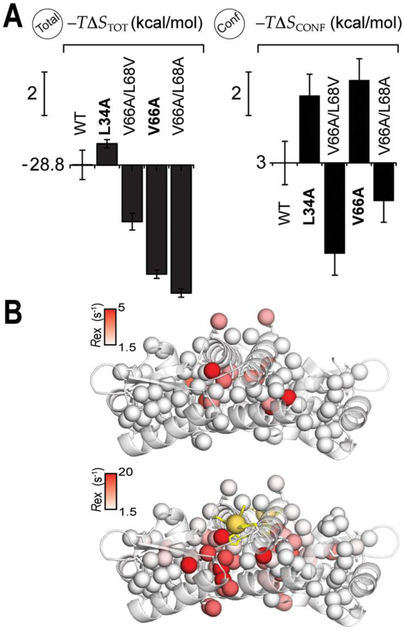
Figure 4.
(A) Graphical illustration of the entropy terms associated with Zn binding to the wild- type vs CzrA variants derived from calorimetric (total, eq 1; resulting for binding two Zn equivalent per dimer) and internal protein dynamics measurements (, Conf, eq 7) by NMR spectroscopy. The vertical scale bar corresponds to a change of 2.0 kcal mol−1. The variants marked in bold show conformational exchange in the Zn-bound state. (B) Site-specific methyl Rex in the apo- and Zn2-loaded in L34A CzrA homodimers mapped onto the structure of apo- and Zn2-loaded, respectively.