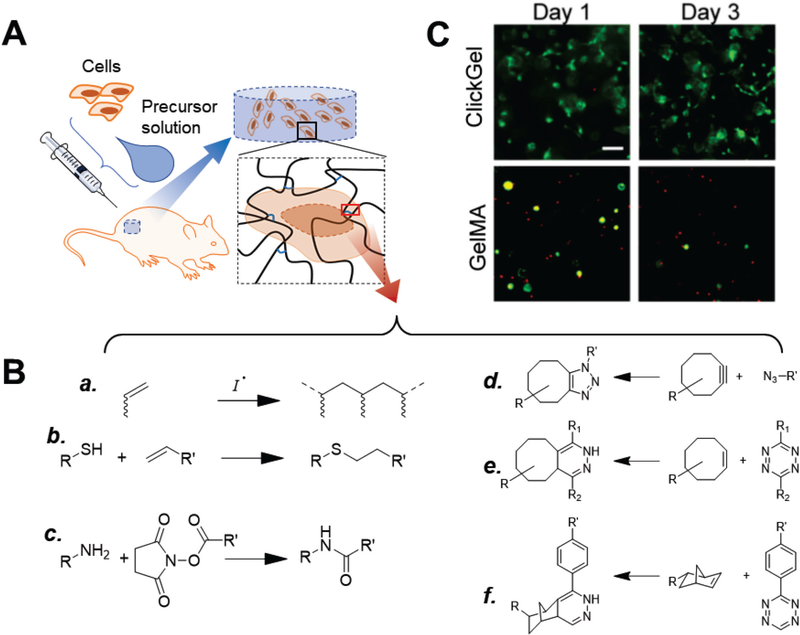
Figure 1.
In-situ hydrogels and crosslinking chemistry. (A) Typical use of in-situ hydrogels for cell delivery. Cells are mixed with hydrogel precursor solution (usually a solution of functionalized polymers) and co-injected into the defect site, where the polymers crosslink into a polymer network as hydrogel scaffolds that support cells. (B) Crosslinking in-situ hydrogels. Conventional chemical reactions used for in-situ hydrogels include (a) photo- and thermal-initiated radical polymerization of (meth)acrylate groups, (b) thiol-ene reaction, and (c) amine-N-hydroxylsuccimide ester coupling.[26] To avoid interference with biological processes, recent studies have explored the use of biorthogonal click reactions, such as (d) strain-promoted azide-alkyne cycloaddition[29–30], (e) tetrazole-alkene[31], and (f) tetrazine-norbornene chemistry[32]. (C) Gelatin hydrogels crosslinked via tetrazine-norbornene click chemistry (ClickGel) led to significantly improved short and long-term cell viability versus radical-crosslinked methacrylated gelatin (GelMA). [32a] Reproduced with permission. [32a] Copyright 2016, John Wiley and Sons.