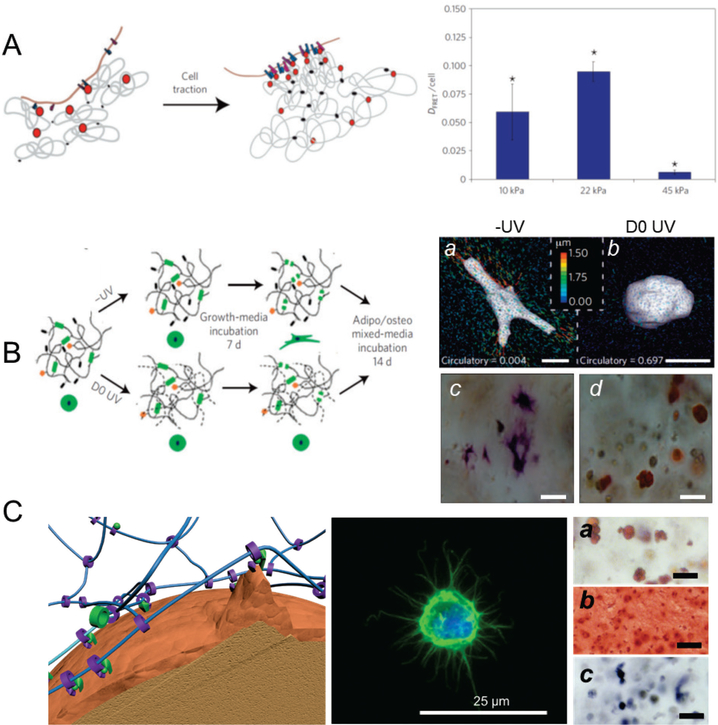
Figure 3.
Molecular mobility enables stem-cell functions in 3D environments. (A) Stem cells perform their functions in 3D in response to the mechanical properties of the matrix, mediated by the manipulation and nanoscale reorganization of surrounding adhesion ligands. Left, Schematic demonstrating cell traction mediated nanoscale clustering of surrounding adhesion ligands (RGD) incorporated in the calcium ion crosslinked alginate hydrogels enhanced cell–RGD bond formation. Right, alginate is incorporated with RGD-FITC and RGD-TAMRA to measure ligand clustering using fluorescence resonance energy transfer. Human MSCs encapsulated in hydrogels demonstrated cell traction-mediated and mechanical stiffness-dependent RGD clustering. Ligand clustering peaked at 22 kPa, consistent with the peak of osteogenic differentiation in this study. (n=3–5; *p<0:01 compared with other conditions, Holm–Bonferroni test) [10a] Reproduced with permission. [10a] Copyright 2010, Nature Publishing Group. (B) Degradation is essential for covalently crosslinked chemical hydrogels to rescue differentiation, since the lack of molecular mobility of covalent bonds restricts cells from reorganizing the niche matrix and experiencing traction. Left, schematic of crosslinking of hyaluronic acid hydrogels using MMP-cleavable peptide only (-UV) and UV initiated radical polymerization of methacrylate groups (D0 UV). Right, representative 3D TFM images (a,b) and bright-field images of hMSC staining for ALP (osteogenesis, c) and lipid droplets (adipogenesis, d). Hyaluronic acid hydrogels crosslinked by MMP-leavable peptide without UV (-UV) allowed encapsulated human MSCsto exert traction, and to change their morphology (right), leading to osteogenic differentiation favored by the matrix stiffness of 4.3 kPa. However, in the presence of the non-degradable UV crosslinked hydrogel counterpart (D0 UV), cells did not reorganize the matrix and remained spherical, with adipogenic differentiation predominating. (Scale bar: a,b 15 μm; c,d 20 μm) [11] Reproduced with permission. [11] Copyright 2013, Nature Publishing Group. (C) Mobile ligands and crosslinks in sliding hydrogels provide essential molecular mobility without introducing degradation. Left, ligands and crosslinks are covalently attached to cyclodextrin rings that are confined on the linear PEG chains, with the ability to slide and rotate. Molecular mobility enables encapsulated human MSCs to reorganize the surrounding ligands and (middle) to change their morphology to form protrusions. Right, representative images of hMSCs in a sliding hydrogel stained for (a) neutral lipid accumulation (Oil Red O; adipogenesis), (b) glycoaminoglycan deposition (Safranin O, chondrogenesis), and (c) ALP activity (Fast Blue; osteogenic) after culture in adipogenic, chondrogenic, and osteogenic medium. Without changing the mechanical properties of the sliding hydrogels, human MSCs differentiate toward multiple lineages including adipogenesis, chondrogenesis, and osteogenesis. (Scale bar a-c, 50 μm). [12] Reproduced with permission. [12] Copyright 2016, John Wiley and Sons.