Figure 5.
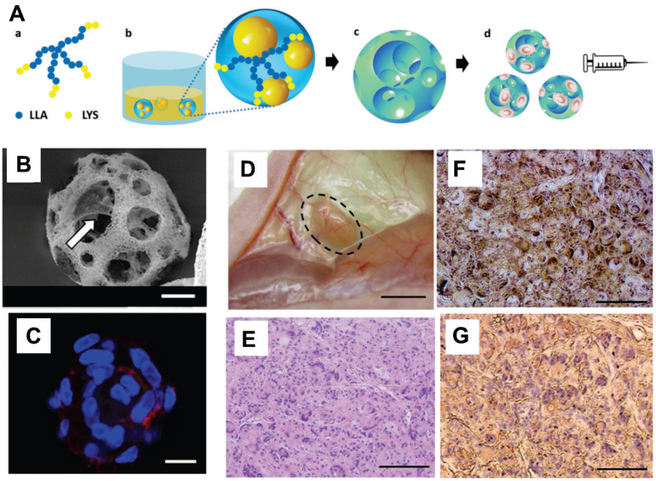
Nanofibrous spongy microspheres are injectable, porous, cell-delivery matrices that enhance cell retention and proliferation in vivo. (A) Scheme of fabrication of these microspheres. Star-shaped poly(L-lactic acid)-block-polylysine (SS-PLLA-b-PLYS) copolymer (a) is used to form microspheres using a reversed emulsion method (b) and phase separation-freeze drying (c). These porous microspheres can be loaded with cells for delivery via injection (d). (B) Scanning electron microscopy reveals that the microporous structure of these spheres facilitates cell attachment to the surface of the inner pores (arrow). (C) Dental pulp stem cells were well distributed in porous microspheres, with high coverage of the inner surface. Cells were stained with Alexa Fluor 555 phalloidin (F-actin, red) and DAPI (nuclei, blue). (D) Odontogenic differentiation of human dental pulp stem cells in vivo 6 weeks after subcutaneous injection to demonstrate the dentin tissue regeneration using the nanofibrous spongy microspheres as carrier. Cell-loaded spheres were pre-cultured in vitro for 7 days. (E) Hematoxylin and eosin staining indicated substantial tissue formation and high cell density, with even distribution. (F) von Kossa staining demonstrated enhanced mineralization. (G) Nanofibrous spongy microspheres promoted odontogenic differentiation of human dental pulp stem cells, as indicated by immunohistological staining for dentin sialophosphoprotein. (Scale bar: B,C, 10 μm; D, 2 mm; E-G, 100 μm)
(A-G) Reproduced with permission. [53b] Copyright 2015, John Wiley and Sons.