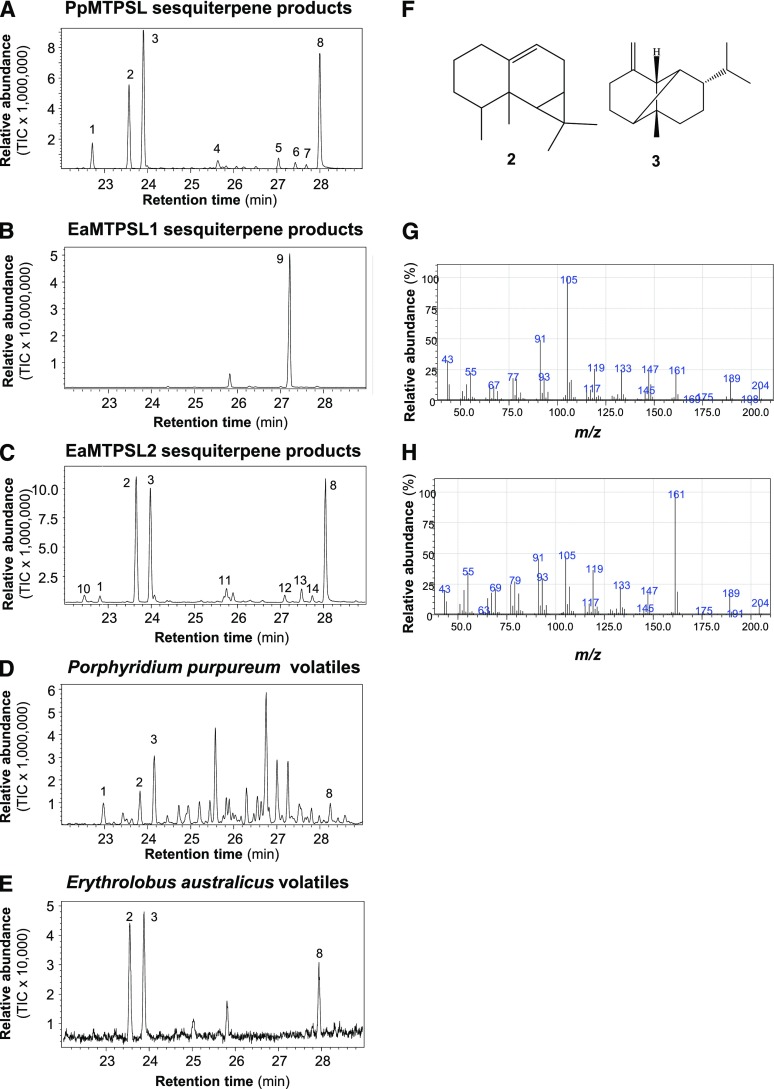
Figure 4.
Red algal MTPSLs have sesquiterpene synthase activities, and their in vitro terpene products are emitted as volatiles. A, Sesquiterpene products formed from recombinant PpMTPSL. B, Sesquiterpene products formed from recombinant EaMTPSL1. C, Sesquiterpene products formed from recombinant EaMTPSL2. D, Sesquiterpene volatiles emitted from P. purpureum. E, Sesquiterpene volatiles emitted from E. australicus. All samples were analyzed by gas chromatography-mass spectrometry (GC-MS), and the total ion chromatograms (TIC) are shown. 1, β-Elemene; 2, aristol-9-ene; 3, β-copaene; 4, germacrene A; 5 to 7, unidentified sesquiterpenes; 8, unidentified oxygenated sesquiterpene (Kovats retention index = 1,593); 9, nerolidol; 10, α-ylangene; 11, α-muurolene; 12, unidentified sesquiterpene; 13, (Z)-α-bisabolene epoxide; 14, unidentified sesquiterpene. β-Elemene is an artifact of germacrene A degradation during GC analysis. F to H, Structures (F) of compounds 2 (aristol-9-ene) and 3 (β-copaene), along with their mass spectra (G and H, respectively).