Fig. 3. Comparison of closed and open conformations of 6hisHpUreI elucidates coordinated transitions involving interprotomer cooperation.
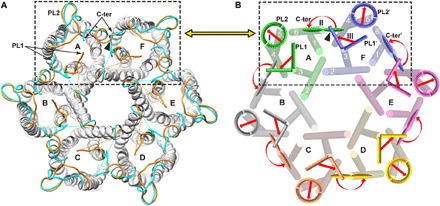
(A) Periplasmic view of superposed hexamers in closed and open conformations, with their PL1, PL2, and C terminus highlighted in orange (closed) and cyan (open). (B) Illustration of sequential cooperative changes in one protomer (A) that would convert a neighboring protomer (F) from closed to open conformation (black dashed boxes). Our structures show that steric hindrance (black triangle) would prevent the simultaneous occurrence of the C terminus (C-ter) of protomer A in its closed conformation and PL1′ of protomer F in its open conformation. We posit that, as indicated by arrows, the movement of PL2 on protomer A (step I) allows its C terminus to flip 180° (step II), which makes space for PL1′ of the neighboring protomer F to reorient to the open conformation (step III). The final open state of protomer F (i.e., the open channel structure presented here) would then be formed with conversion of its own PL2 (PL2′) and C terminus (C-ter′) to the open conformation.
