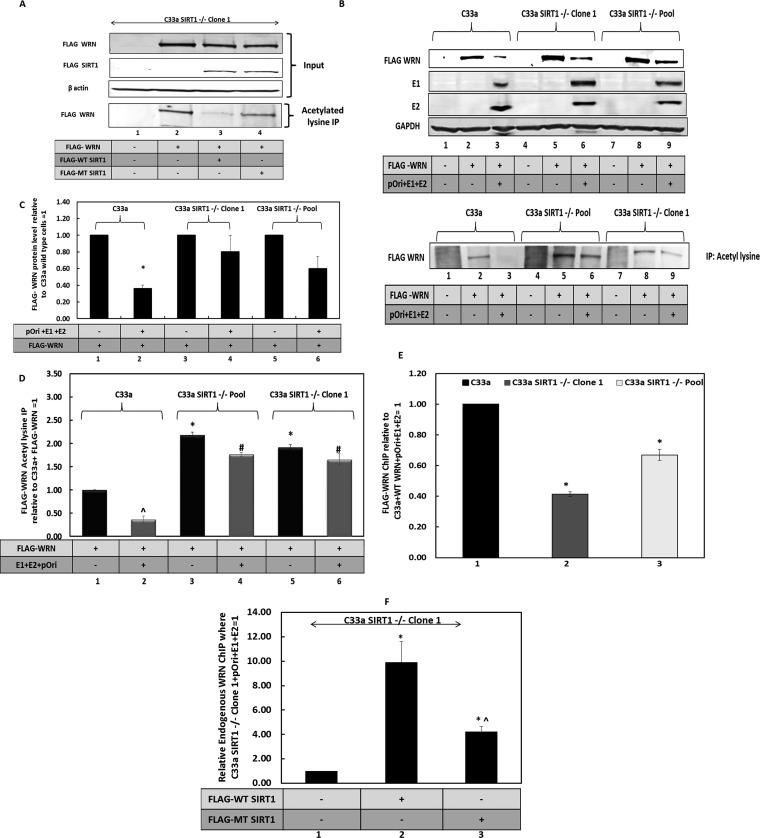
FIG 3.
SIRT1 regulates the acetylation status of WRN. (A) FLAG-WRN, FLAG-WT SIRT1 (wild type), and FLAG-MT SIRT1 (deacetylase mutant) were coexpressed in C33a cells. The upper blots show the input levels of the proteins. The lower blot demonstrates the levels of FLAG-WRN immunoprecipitated by an acetyl lysine residue antibody. FLAG-WT SIRT1 reduces the levels of FLAG-WRN acetylation (compare lane 3 with lane 2), while FLAG-MT SIRT1 is compromised in this property (compare lane 4 with lane 3). (B) FLAG-WRN levels are reduced by the E1-E2 replication complex. In wild-type C33a cells, FLAG-WRN levels are reduced by the E1-E2 replication complex (compare lane 3 with lane 2). However, in C33a SIRT1–/– clone 1 and pool cells, there is not as pronounced a reduction in FLAG-WRN levels (compare lanes 6 and 9 with lane 3). These extracts were immunoprecipitated with an acetyl lysine antibody and subjected to Western blotting for FLAG-WRN (lower panel). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) Quantitations of FLAG-WRN for the input blot in panel B are represented graphically and represent the summary of results from three independent experiments. There is a significant reduction in FLAG-WRN levels in wild-type C33a cells in the presence of the E1-E2 replication complex (P value was less than 0.05), and this reduction is largely lost in the absence of SIRT1 (compare lanes 4 and 6 with lane 2; standard error bars are shown). The results are expressed relative to levels in the absence of E1-E2, which equals 1 in each of the cell types. (D) The results obtained from the acetyl lysine IP experiments shown in the lower section of panel B were quantitated. ^ indicates a significant reduction in acetylated FLAG-WRN in the presence of the E1-E2 replication complex in C33a wild-type cells. * indicates an increase in FLAG-WRN acetylation in the absence of replication or a reduction of SIRT1 relative to the levels observed in wild-type C33a cells. # indicates an increased acetylation of FLAG-WRN in the presence of the E1-E2 replication complex but in the absence of SIRT1, compared with that of wild-type C33a cells. The significance is determined by a P value of less than 0.05, and standard error bars are shown. The results represent a summary of two independent experiments. (E) Even though there are elevated FLAG-WRN levels in the absence of SIRT1, there is a failure of recruitment of this protein to E1-E2-replicating DNA, as demonstrated by ChIP. The results are presented relative to those of the FLAG-WRN ChIP for wild-type C33a cells (lane 1), and there is a clear reduction in the presence of this protein on E1-E2-replicating DNA in the absence of SIRT1 (lanes 2 and 3). This difference is significant (*; P value was less than 0.05; standard error bars are shown). Results represent a summary of at least 3 independent experiments.