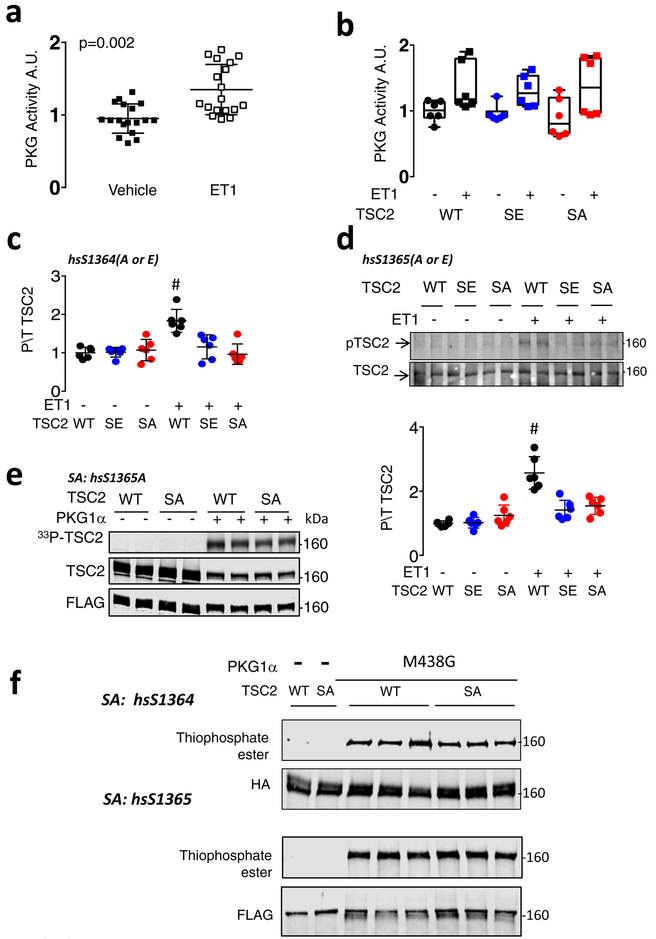
Extended Data Figure 3: PKG is activated by endothelin-1 in myoyctes, and results in TSC2 phosphorylation detected by hsS1364-Ab in cells expressing WT but not huS1364A or huS1365A mutations. Direct PKG-TSC2 phosphorylation is confirmed using recombinant proteins ± SA mutations, and in cell lysates.
a) PKG activation in myocytes expressing WT, SA, or SE TSC2 and stimulated with endothelin-1 (ET1, 10 nM) versus vehicle for 48 hours. Mean±SD, n=18 biologically independent samples, unpaired Student’s 2-tailed t-test. b) PKG activation is independent of the form of TSC2 expressed; n=6 biologically independent samples, box/whisker and raw data plot; p=0.0004 for ET-1 effect, p>0.8 for group effect by 2-way ANOVA. c) Summary data for phospho/total S1364 TSC2 (human sequence) from ET1-stimulation experiment shown in Figure 1f. Mean±SEM, n=6 biologically independent samples from 3 experiments, 1WANOVA, Tukey multiple comparisons test, # p=0.003 vs SE, p=0.0003 vs SA. d) Antibody raised to mmS1365 (mouse) (equivalent to huS1364, human) shows increase TSC2 phosphorylation in myocytes transfected with WT TSC2 plasmid, but not cells expressing TSC2 SA or SE mutations at muS1366 (huS1365). The experiment is replicated x3, with n=6 biologically independent samples, Mean±SD. 1WANOVA, Tukey multiple comparisons test, # p=0.0002 vs SE, p=0.0007 vs SA. The results are identical to those using muS1365 (hsS1364) mutants, indicating that mutating either serine (SA or SE) prevents phosphorylation of the other and/or its detection by the phospho-antibody. e) Direct TSC2 phosphorylation by recombinant PKG1α detected by autoradiography on hsTSC2-FLAG (WT) and hsTSC2-FLAG-S1365A. Data replicated × 3; n=6 biologically independent samples, with identical results. The result is identical to that in Figure 1j with hsTSC2-HA-S1364A. f) Direct TSC2 phosphorylation by PKG1α in lysates from TSC2-KO HEK cells expressing WT or hsTSC2-HA-S1364A or hsTSC2-FLAG-S1365A, PKG1α M438G, and N6-Benzyl-ATPγS, and probed for thiophosphate ester. Top gel shows data with huS1364 mutated, lower with huS1365 mutated. The results are identical. n=6 biologically independent samples for each assay.