Abstract
Occupational exposure to 1-bromopropane (1-BP) induces learning and memory deficits. However, no therapeutic strategies are currently available. Accumulating evidence has suggested that N-methyl-D-aspartate receptors (NMDARs) and neuroinflammation are involved in the cognitive impairments in neurodegenerative diseases. In this study we aimed to investigate whether the noncompetitive NMDAR antagonist MK801 protects against 1-BP-induced cognitive dysfunction. Male Wistar rats were administered with MK801 (0.1 mg/kg) prior to 1-BP intoxication (800 mg/kg). Their cognitive performance was evaluated by the Morris water maze test. The brains of rats were dissected for biochemical, neuropathological, and immunological analyses. We found that the spatial learning and memory were significantly impaired in the 1-BP group, and this was associated with neurodegeneration in both the hippocampus (especially CA1 and CA3) and cortex. Besides, the protein levels of phosphorylated NMDARs were increased after 1-BP exposure. MK801 ameliorated the 1-BP-induced cognitive impairments and degeneration of neurons in the hippocampus and cortex. Mechanistically, MK801 abrogated the 1-BP-induced disruption of excitatory and inhibitory amino-acid balance and NMDAR abnormalities. Subsequently, MK801 inhibited the microglial activation and release of pro-inflammatory cytokines in 1-BP-treated rats. Our findings, for the first time, revealed that MK801 protected against 1-BP-induced cognitive dysfunction by ameliorating NMDAR function and blocking microglial activation, which might provide a potential target for the treatment of 1-BP poisoning.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0321-8) contains supplementary material, which is available to authorized users.
Keywords: 1-Bromopropane, Cognitive dysfunction, MK801, N-methyl-D-aspartate receptors, Microglia, NLRP3 inflammasome
Introduction
Chlorofluorocarbons have been implicated in the depletion of the stratospheric ozone layer, and also increase the incidence of cataract formation and skin cancer [1]. As an alternative to ozone-depleting solvents, 1-bromopropane (1-BP; Chemical Abstracts Service Registry No. 106-94-5) is widely used in industry, for example in spray adhesives, metal and electronic component cleaning, and dissolving fats, waxes, or resins [2, 3]. With the expanded application, the adverse effects of 1-BP exposure have gained increasing attention. Studies in experimental animals and workers have revealed severe toxic effects of 1-BP in the central nervous system [4–6]. We recently reported that 1-BP poisoning in rats results in learning and memory deficits and hippocampal neuronal damage [7, 8]. Workers exposed to 1-BP display depression, anxiety, reduced short-term memory, headaches, confusion, and loss of consciousness [9–12]. Cognitive impairments occur in patients poisoned by 1-BP, and these have gradually been recognized to be key factors that decrease their quality of life [13]. However, therapeutic strategies aimed at blocking 1-BP-induced cognitive impairments and the related neurodegeneration remain to be investigated.
N-methyl-D-aspartate receptors (NMDARs) are glutamate-gated Ca2+ channels that play pivotal roles in physiological and pathological conditions, such as neuronal communication and synaptic plasticity [14–16]. In the brain, NMDARs are expressed in neurons and glial cells, including microglia, astrocytes, and oligodendrocytes [17]. Activation of NMDARs contributes to the induction of long-term potentiation [18]. However, dysregulated activation of NMDARs may trigger neuronal death and associated cognitive impairments in neurodegenerative disorders, such as schizophrenia and Alzheimer’s disease (AD) [15, 19–22]. Pioneering studies reported that exposure to 1-BP increases the neuronal excitotoxicity in the CA1 and the dentate gyrus (DG) areas of the hippocampus, and this is suppressed by an NMDAR antagonist [23], indicating the involvement of NMDARs in 1-BP-induced neurotoxicity.
Neuroinflammation mediated by microglia has been identified as a key factor driving the neurodegenerative process in many neurological disorders [24, 25]. Activated microglia generate a detrimental microenvironment for neurons by secreting a variety of factors, such as reactive oxygen/nitrogen species, prostaglandins, and inflammatory cytokines, which work in concert to cause neuronal damage [26, 27]. Recent studies have suggested cross-talk between NMDARs and microglial activation. Chronic NMDA administration increases the protein and mRNA levels of pro-inflammatory factors, including inducible nitric oxide synthase, interleukin-1beta (IL-1β), and tumor necrosis factor alpha (TNFα) in rat frontal cortex [19]. Moreover, MK801 and dextromethorphan, two widely-used NMDAR antagonists, inhibit microglial activation and protect against methamphetamine-elicited neurotoxicity [27, 28]. Studies have shown that 1-BP exposure is capable of inducing the activation of microglia in rat cerebellum in vivo and promoting the release of pro-inflammatory factors in mouse macrophages in vitro [29, 30]. However, whether the disruption of immune homeostasis induced by 1-BP is related to the activation of NMDARs remains unknown. Therefore, in this study, we gave Wistar rats the noncompetitive NMDAR antagonist MK801 prior to 1-BP intoxication. The effects of MK801 on 1-BP-induced cognitive dysfunction, NMDAR subunit expression and phosphorylation status, microglial activation, and the production of pro-inflammatory cytokines in the hippocampus and cerebral cortex were investigated.
Materials and Methods
Reagents
1-BP (≥ 99.99% purity) was from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). MK801 and 3,3-diamino-benzidine-tetrachloride (DAB) with metal enhancer were from Sigma-Aldrich Chemical Co. (St. Louis, MO). The monoclonal antibodies anti-GluN1 (ab109182), anti-GluN2A (ab124913), anti-phospho-GluN2A (p-GluN2A, ab16646), anti-phospho-GluN2B (p-GluN2B, ab81271), and anti-NLRP3 (ab4207), and polyclonal anti-GluN2B (ab65783) were from Abcam (Cambridge, UK). Anti-caspase1 antibody (sc56036) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and anti-IL-1β (12507) from Cell Signal Technology (Danvers, MA). Anti-ionized calcium-binding adaptor molecule-1 (Iba-1) was from Wako Pure Chemical Industries, Ltd. The monoclonal anti-neuronal nuclei (NeuN) (cat# MABA377) was from Millipore (Darmstadt, Germany). β-Actin monoclonal antibody (clone AC-15) was supplied by Sigma. The BCATM protein assay kit was from Pierce Biotechnology (Rockford, IL). Secondary antibodies horseradish peroxidase (HRP)-linked IgG and biotin-conjugated IgG were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The chemiluminescence detection kit for HRP was from Biological Industries Israel Beit Haemek Ltd. (Kibbutz Beit Haemek, Israel). Polyvinylidene difluoride (PVDF) membranes were from Merck Millipore (Billerica, MA). All chemicals used were of the highest grade commercially available.
Animal Treatment
The entire study was conducted according to protocols approved by the Ethics Committee for Animal Experiments of the School of Public Health, Shandong University, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Forty-eight specific pathogen-free male Wistar rats (body weight 220 g–240 g) were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All rats were housed and maintained under controlled conditions with a 12 h/12 h light/dark cycle (lights on at 08:00 and off at 20:00) at 22 ± 2°C and 50%–60% relative humidity. Drinking water and commercial animal feed were provided ad libitum.
After 5 days of acclimatization to the new environment, the rats were randomly divided into four groups (n = 12/group) as follows: control, 1-BP, MK801 + 1-BP, and MK801 groups. 1-BP was dissolved in corn oil and orally administered at 800 mg/kg body weight for 14 consecutive days, based on previously-published data [7, 8, 31]. In the MK801 + 1-BP group, MK801 (0.1 mg/kg body weight) was injected intraperitoneally (i.p.) 30 min prior to 1-BP for consecutive 14 days. The dose of MK801 was chosen according to previous reports [27, 28]. In the control group, rats were orally given an equivalent volume of corn oil. In the MK801 group, rats were injected with MK801 (0.1 mg/kg body weight, i.p.) alone. Cognitive dysfunction was assessed in the Morris water maze (MWM) from experimental day 10 to day 14. At the end of the MWM test, 8 randomly-selected rats from each group were sacrificed by decapitation after 5% chloral hydrate anesthesia. The cerebellum was dissected out, then the cerebral cortex and hippocampus were immediately separated on ice, frozen in liquid nitrogen, and stored at − 80°C until use. The remaining rats in each group were anesthetized with intraperitoneal injection of 5% chloral hydrate and perfused intracardially with saline followed by a solution of 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer saline (PBS). The brains were used for morphological assessment. The body weight was recorded on days 1, 7, and 14.
Morris Water Maze Tests
The MWM was used to measure spatial learning and memory. The apparatus and protocols were as previously described [7, 8, 32]. The movement path was recorded and analyzed using a computerized tracking system consisting of a camera and video-tracking software (Huaibei Zhenghua Biological Instrument Equipment Co., Ltd., Suixi, China). The rats were tested on experimental days 10–14.
Spatial Navigation Test
Throughout the spatial navigation test, the platform was always located in the south-west quadrant. The rats received four daily trials for 4 consecutive days. During a trial, animals were gently put into the water facing the pool wall in one of the four quadrants, and allowed to swim until they reached the platform. They were given 120 s to find the hidden platform, and if they failed to find the platform within 120 s, the rats were guided to the platform by the tester and the latency recorded as 120 s. Between trials the rats remained on the platform for 20 s. The swimming times and distances to find the platform are referred to as the escape latency and swimming distance, respectively, and were used to evaluate spatial learning.
Spatial Probe Trial
To assess spatial memory, a probe test was performed on day 5 after the 4 days of spatial navigation tests. During the probe trial, the hidden platform was taken away, and rats were released into the water in each quadrant for a 90-s free-swimming period, with a break of 20 s in a clean cage between trials. The number of times of the rat crossing the previous location of the platform and the percentage of time spent in the original platform quadrant were recorded and analyzed using tracking software.
Analysis of Amino-Acids
High performance liquid chromatography (HPLC) was used to evaluate changes in the levels of the amino-acids γ-amino butyric acid (GABA), aspartate (Asp), glutamate (Glu), glycine (Gly), and taurine (Tau) in the cerebral cortex and hippocampus. This was based on previously-published methods that are highly reproducible and have been validated in terms of accuracy, precision, linearity, sensitivity, and stability [33].
Briefly, selected brain tissues were extracted, weighed, and added to HPLC-grade water-methanol solution (m:v = 1:9), then the samples were homogenized for 90 s by a biological homogenizer (AO Sheng Equipment Co., Ltd., Hangzhou, China). The homogenates were centrifuged at 12000 g for 10 min at 4°C and the supernatant was filtered through a 0.22 µm filter. The standard solution or sample solution was reacted with NaHCO3 and 4-fluoro-7-nitrobenzofurazan, and then injected into the HPLC system. HPLC was performed using a Waters 2998 Photodiode Array Detector (Waters Corporation, Milford, MA). The HPLC separation was achieved using a C18 column (5 μm, 250 mm × 4.6 mm) maintained at 40°C, and the amino-acid components were eluted by gradient elution with 0.5 mol/L sodium acetate solution (solvent A), acetonitrile (solvent B), and water (solvent C). The gradient was as follows: 88% A, 88% B, 6% C at 0 min; 65% A, 17.5% B, 17.5% C at 5.0 min; 40% A, 30% B, 30% C at 14.0 min; and 88% A, 6% B, 6% C at 25 min. The flow rate was 1.0 mL/min and the UV detection wavelength was set at 350 nm.
Histological and Immunohistochemical Analysis
Four rats from each group were anesthetized by i.p. injection of 4% chloral hydrate and intracardially perfused with 0.9% saline, followed by 4% paraformaldehyde (PFA, ice-cold) in 0.1 mol/L phosphate buffered saline (PBS, pH 7.4). The brain was removed and fixed in 4% PFA (4°C) for 48 h, transferred to 30% sucrose, and then left until it sank to the bottom. Coronal frozen sections (40 μm) including cerebral cortex and hippocampus were cut on a freezing microtome (HM525, Thermo, MA) and mounted on Thermo SuperFrost Plus slides (Sigma). Two series of adjacent sections (two sections per rat) with similar parts of the cerebral cortex and hippocampus were selected for thionin staining and immunostaining.
Immunostaining was performed as previously described [7, 32]. Briefly, free-floating sections were blocked with 1% bovine serum albumin, 4% normal goat serum, and 0.4% TritonX-100 in PBS for 30 min at room temperature, then incubated with primary anti-NeuN or anti-Iba-1 antibody (1:2000 dilution) overnight at 4°C. The next day, the sections were incubated in biotin-conjugated secondary antibody for 2 h at room temperature. Then the sections were further stained using an ABC Kit (Vector Laboratories, Inc., Burlingame, CA) and DAB with metal enhancer. After mounting on slides, the sections were observed under a light microscope, and images were captured to count the number of NeuN-immunopositive neurons and Iba-1-immuopositive microglia using ImageJ software (NIH, USA).
Western Blot Analysis
The brain samples were weighed and homogenized using a motorized homogenizer in ice-cold lysis buffer containing the following: 50 mmol/L NaCl, 1% Triton X-100, 50 mmol/L HEPES, 0.5% deoxycholate sodium, 0.1% SDS, 2 mmol/L Na3VO4, 1 mmol/L NaF, 1 mmol/L EGTA, 12 mmol/L β-mercaptoethanol, 1 mmol/L PMSF, and 1% protein protease inhibitor cocktail. The homogenate was kept at 4°C for 30 min and then centrifuged at 1,000 g for 15 min. The supernatant was collected and further centrifuged at 12,000 g for 20 min. Then, the protein concentrations in the supernatants were determined with a BCA protein assay kit. Next, the protein samples were mixed with loading buffer and boiled for 10 min. Equal amounts of protein (20 μg) were separated by 7.5% or 10% SDS polyacrylamide gel electrophoresis, then transferred to a PVDF membrane. After blocking with 5% skim milk in TBST for 1 h at room temperature, the membranes were incubated with the primary antibodies anti-β-actin, anti-GluN1, anti-GluN2A, anti-GluN2B, anti-p-GluN2A, anti-p-GluN2B, anti-NLRP3, anti-caspase1, and anti-IL-1β overnight at 4°C. Then the membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature (1:5000). After washing, the proteins were visualized using the chemiluminescence method. The bands on X-ray film were scanned, then integral optical density analysis of proteins was performed with the Kodak Imaging Program and Image-Pro Plus (Eastman Kodak Co., New Haven, CT), with normalization to β-actin.
Statistical Analysis
All data are presented as mean ± SD and were analyzed using the Statistical Package for Social Sciences (SPSS) version 23 (SPSS Inc., Chicago, IL). The data from the MWM test and body weight were analyzed by repeated-measures analysis of variance (ANOVA). Other data were compared using one-way ANOVA followed by the LSD post-hoc test. P < 0.05 was considered statistically significant.
Results
Changes in Rat Body Weight
To investigate the effect of MK801 on the physical development of 1-BP-treated rats, the body weight was recorded at 1, 7, and 14 days after initial 1-BP intoxication with or without MK801 pre-treatment. The body weight of all groups gradually increased (Table 1). At 14 days, the weight had increased by 30.92% in the control group, and this was reduced to 20.38% in 1-BP-intoxicated rats. Interestingly, the weight in rats with combined MK801 and 1-BP treatment recovered to a level comparable to controls (27.75% ± 3.85%). MK801 alone did not affect the body weight gain compared to control rats. No deaths were recorded during experiments.
Table 1.
Body weight gain in the four groups
| Group (n = 12) | Body weight (g) | Body weight gain (%) | ||
|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | ||
| Control | 237.25 ± 8.40 | 284.00 ± 17.17 | 310.42 ± 10.57 | 30.92 ± 4.78 |
| 1-BP | 241.85 ± 9.06 | 267.54 ± 10.37 | 291.08 ± 13.07 | 20.38 ± 3.82** |
| 1-BP + MK801 | 238.92 ± 8.45 | 274.83 ± 10.74 | 305.25 ± 14.76 | 27.75 ± 3.85## |
| MK801 | 238.25 ± 8.08 | 280.75 ± 11.54 | 313.50 ± 14.63 | 31.63 ± 5.51 |
**P < 0.01 compared to control; ##P < 0.05 compared to 1-BP group
MK801 Treatment Ameliorated the 1-BP-Induced Impairments in Spatial Learning and Memory
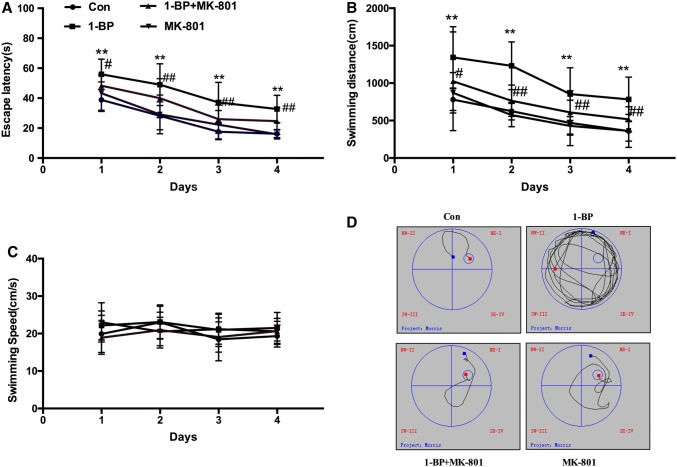
In the place navigation test, the performance of all groups improved as the training continued. Compared with the control group, 1-BP exposure markedly increased the escape latency and swimming distance (Fig. 1A, B), which were significantly reduced by pre-treatment with MK801, indicating that MK801 effectively improved the learning and memory of 1-BP-treated animals. Consistently, the 1-BP rats showed a random type of underwater platform searching profile, the control rats exhibited an effective exploratory profile, while co-administration of MK801 significantly improved the 1-BP-induced change in exploratory profile (Fig. 1D). Administration of MK801 alone had no effect on memory compared to the control group. There were no significant differences in swimming speed among the groups (Fig. 1C).
Fig. 1.
MK801 protects against 1-BP-induced spatial learning deficits in rats. The spatial learning of rats intoxicated with 1-BP with or without MK801 pre-treatment was tested using the Morris water maze. A Time to find the platform (escape latency). B Total distance to find the platform (swimming distance). C Average velocity among groups (swimming speed). D Typical exploratory profiles of rats in the different groups. n = 12, *P < 0.05, **P < 0.01 vs control group; ##P < 0.01 vs 1-BP group.
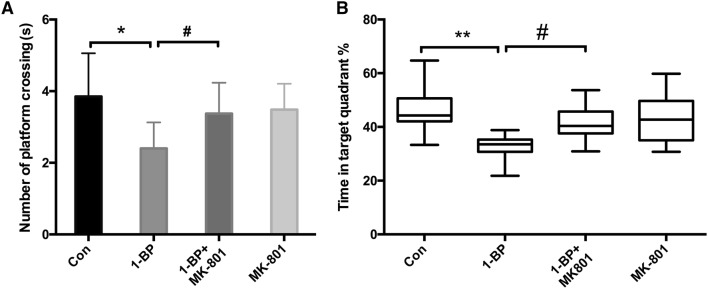
In the probe trial, the platform was removed from the maze, and the rats were allowed to swim freely for 90 s. Compared with the control group, 1-BP exposure significantly reduced the number of platform crossings, which was recovered by pre-treatment with MK801 (Fig. 2A). Rats in the 1-BP group spent a smaller percentage of time in the quadrant with the platform than those in the control group, while pre-administration of MK801 resulted in longer time in the target quadrant than the 1-BP alone group (Fig. 2B).
Fig. 2.
MK801 protects against 1-BP-induced spatial memory impairments in rats. The spatial memory of rats intoxicated with 1-BP with or without MK801 pre-treatment was tested using the Morris water maze. A Number of platform crossings. B Percentage of time spent in the target quadrant. n = 12, *P < 0.05, **P < 0.01, vs control group, #P < 0.05, vs 1-BP group.
MK801 Attenuated 1-BP-Induced Neuronal Damage
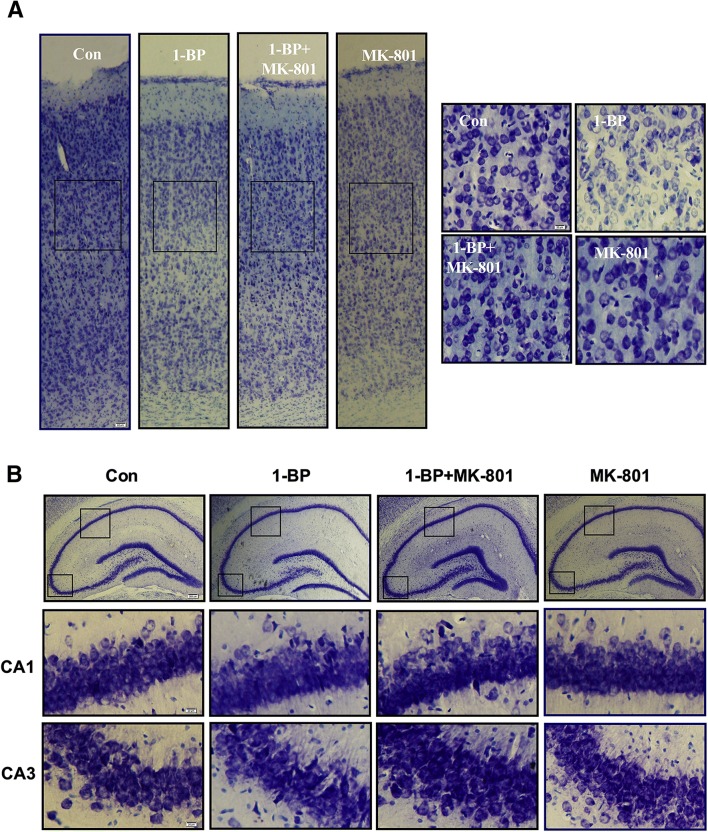
Consistent with the cognitive dysfunction, 1-BP exposure damaged neurons in rats (Fig. 3). Thionin staining revealed swollen and sparse cells in both the hippocampus and cortex of 1-BP-intoxicated rats compared with vehicle controls. In addition, 1-BP treatment resulted in smearing of the cell layers in the hippocampus, especially in the CA1 and CA3 areas (Fig. 3B). In contrast to 1-BP alone, combined MK801 and 1-BP treatment restored neurons in cerebral cortex and hippocampus.
Fig. 3.
MK801 reverses 1-BP-induced morphological changes of neurons in the cerebral cortex and hippocampus of rats. A Representative images of thionin staining in the cortex in the different groups (right panels, magnified images). B Representative images of thionin staining in the hippocampus in the different groups (lower panels, magnified images of CA1 and CA3). Scale barsA left, 50µm; right, 20µm; B upper, 200µm; bottom CA1 and CA3, 20µm.
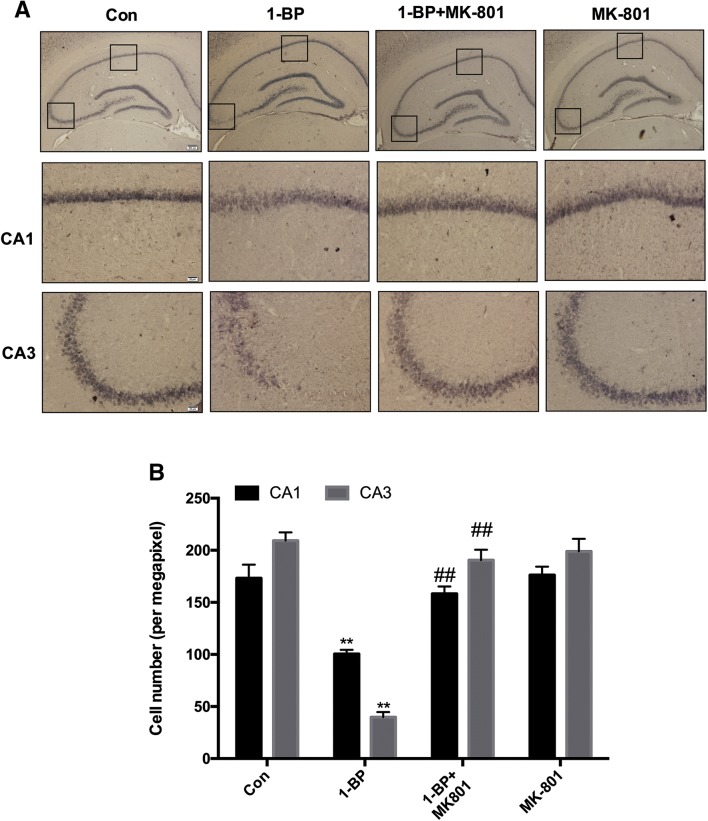
To further confirm 1-BP-induced cell loss in the hippocampus, we performed immunohistochemical staining using the neuron-specific antibody NeuN. The density of NeuN immunostaining in the CA1 and CA3 regions of the hippocampus in the 1-BP-treated rats was significantly down-regulated (Fig. 4A). Measurement of the numbers of NeuN-immunoreactive cells revealed 42.3% loss of neurons in CA1 and 81.0% loss in CA3 in 1-BP-treated rats compared with the control group (Fig. 4B). Interestingly, the neuronal loss in CA1 and CA3 in 1-BP-intoxicated rats increased by 36.5% and 79.1%, respectively when they were supplemented with MK801, suggesting that MK801 protects against 1-BP-induced neurotoxicity.
Fig. 4.
MK801 attenuates 1-BP-induced neuron loss in hippocampal CA1 and CA3 in rats. A Representative images of anti-NeuN immunostaining in each group. Scale bars upper, 200µm; bottom CA1 and CA3, 20µm. B Quantitative measurement of NeuN-immunoreactive neurons in each group. n = 4, **P < 0.01 vs control group. ##P < 0.01 vs 1-BP group.
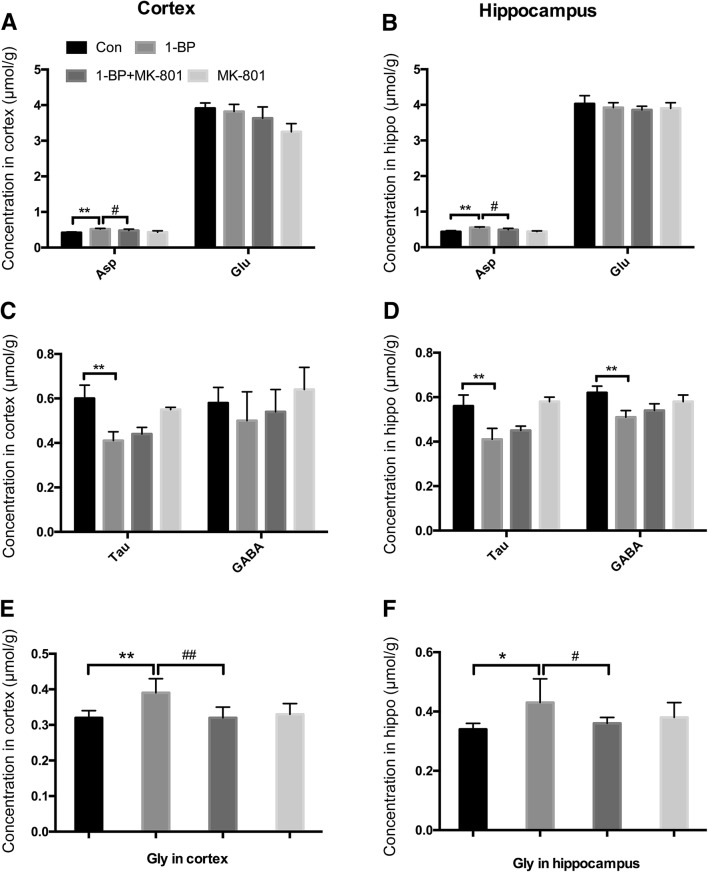
Effects of 1-BP on Amino-Acids in the Brain
To determine the role of NMDARs in 1-BP-induced neurotoxicity, we initially measured the levels of the five amino-acids Asp, Tau, GABA, Glu, and Gly in the hippocampus and cerebral cortex of 1-BP-intoxicated rats with or without MK801 pre-treatment. A mixture of purified Asp, Tau, GABA, Glu and Gly was used to determine the elution time in the HPLC analysis. The results showed that the five amino-acids were completely separated within 15 min (Fig. S1). Specifically, Asp, Glu, Gly, Tau and GABA were separated at 6.105, 7.372, 12.611, 13.410, and 13.865 min, respectively. Exposure to 1-BP significantly increased the levels of Asp and Gly in the hippocampus and cortex compared with vehicle controls (Fig. 5). The levels of GABA in the hippocampus as well as Tau in both the hippocampus and cortex in 1-BP-intoxicated rats were lower than those in the control group. The Glu content remained unchanged. In addition, the 1-BP-induced increases in Asp and Gly were markedly reduced by MK801.
Fig. 5.
MK801 attenuates the 1-BP-induced disruption of excitatory and inhibitory amino-acids in rat hippocampus and cortex. A–D Concentrations of excitatory Asp and Glu (A, B) and inhibitory Tau and GABA (C, D) in each group. E, F Gly levels in each group. n = 4; *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs 1-BP group.
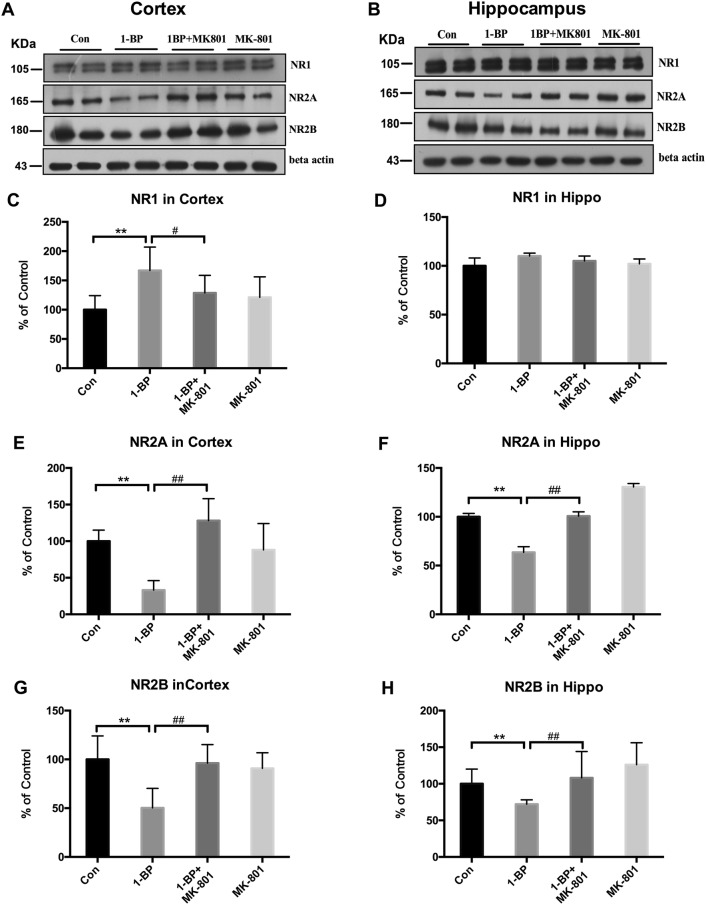
Effects of 1-BP Exposure on Expression of NMDAR Subunits
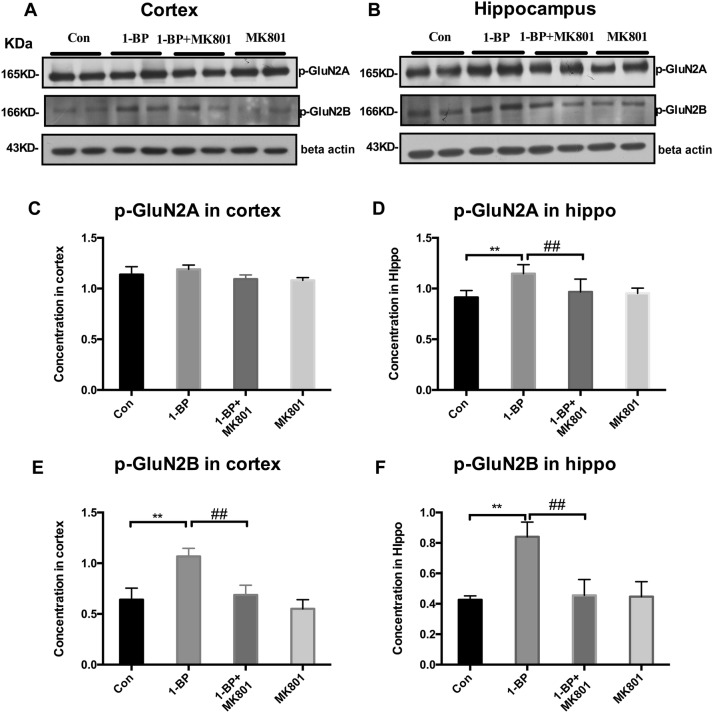
Next, we investigated the effects of MK801 on 1-BP-induced NMDAR subunit expression and NMDAR phosphorylation in both the hippocampus and cortex of rats. Western blots showed that, compared with vehicle controls, the level of the NMDAR subunit GluN1 was significantly increased in the cortex but not the hippocampus of 1-BP-intoxicated rats (Fig. 6C, D). The levels of GluN2A and GluN2B were lower in both the cerebral cortex and hippocampus of the 1-BP group (Fig. 6) than in vehicle controls. In contrast, the phosphorylation of GluN2B was increased in both the cerebral cortex and hippocampus as well as GluN2A in the hippocampus of 1-BP treated rats (Fig. 7). The alterations of NMDAR subunits induced by 1-BP were abrogated by MK801 pretreatment.
Fig. 6.
MK801 attenuates the expression of NMDAR subunits in 1-BP-intoxicated rats. A, B Representative Western blots of the expression of NMDAR subunits GluN1, GluN2A, and GluN2B in hippocampus and cortex of rats in each group (β-actin served as an internal control). C–H Quantification of blot density expressed as a percentage of control (± SEM). n = 4; *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs 1-BP group.
Fig. 7.
MK801 attenuates the expression of phosphorylated GluN2A and GluN2B in 1-BP-intoxicated rats. A, B Representative Western blots of the expression of p-GluN2A and p-GluN2B in hippocampus and cortex of rats in each group (β-actin served as an internal control). C–F Quantification of blot density expressed as a percentage of control (± SEM). (n = 4; *P < 0.05, **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs 1-BP group).
MK801 Treatment Significantly Decreased Activated Microglia and Inhibited Inflammatory Cytokine Release in the Hippocampus and Cerebral Cortex
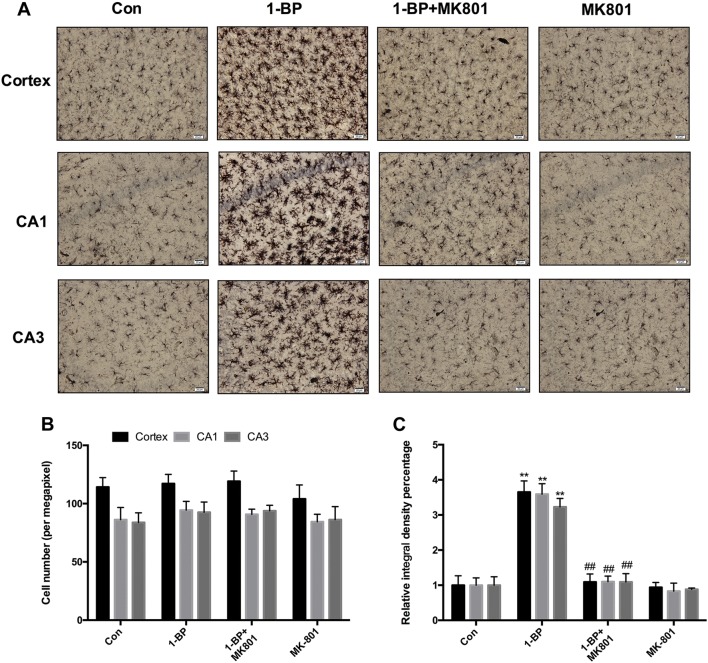
To determine whether the protection afforded by MK801 in 1-BP-intoxicated rats was associated with an attenuation of microglial activation, we performed immunostaining with antibody against Iba1, a microglial marker. The density of Iba-1 staining and the number of activated microglia were analyzed. The activated microglia, characterized by bushy morphology with increased cell body size, along with contracted and ramified processes were observed in the hippocampus and cortex of 1-BP-intoxicated rats. However, such activated microglia were rarely detected in 1-BP-intoxicated rats pretreated with MK801 (Fig. 8A). Analysis of the density of Iba-1 staining supported the morphological observations. The density of Iba-1 staining was increased by 55.2% in cortex, 52.7% in CA1, and 59.3% in CA3 of 1-BP-treated rats compared with vehicle controls; this was significantly reduced by MK801 pretreatment (Fig. 8C). No difference was found in the numbers of Iba-1+ cells among these groups (Fig. 8B).
Fig. 8.
MK801 attenuates 1-BP-induced microglial activation in rats. (A–C) Representative images of anti-Iba-1 immunostaining (A), numbers of Iba-1-immunoreactive cells (B), and integrated density of Iba-1 staining (C) in cortex and hippocampal CA1 and CA3 areas in each group. n = 4, **P < 0.01 vs control group; ##P < 0.01 vs 1-BP group. Scale bars 20µm.
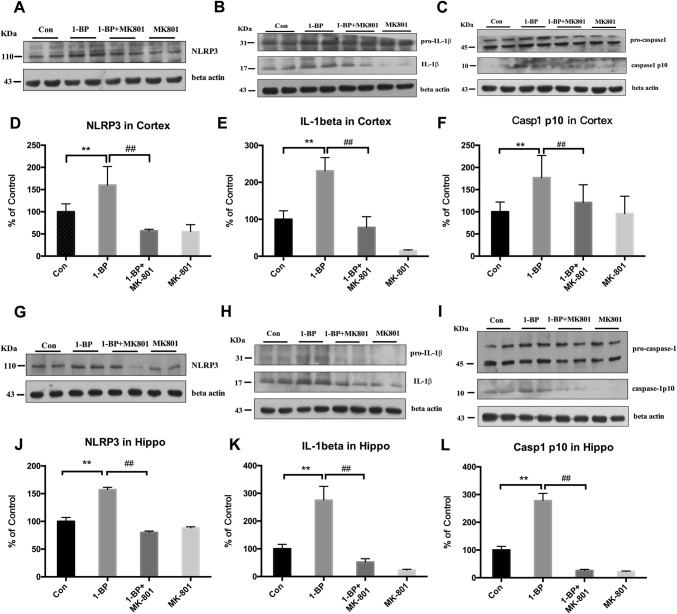
The nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing 3 (NLRP3) inflammasome plays a critical role in regulating microglial activation. To determine whether the inhibitory effects of MK801 on 1-BP-induced microglial activation are associated with inactivation of the NLRP3 inflammasome, the levels of NLRP3, caspase-1, and IL-1β in the hippocampus and cerebral cortex of rats were measured using Western blot. In 1-BP-treated rats, the levels of NLRP3 protein (Fig. 9A, D) were significantly higher than in controls and these effects were inhibited by MK801 pretreatment. Furthermore, Western blot analysis revealed increased levels of mature caspase-1 (casepase-1p10) (Fig. 9C, F) and IL-1β (Fig. 9B, E) in 1-BP-intoxicated rats, which was also mitigated by MK801, suggesting that MK801 attenuates the activation of the NLRP3 inflammasome induced by 1-BP.
Fig. 9.
Effects of 1-BP on NLRP3 inflammasome, IL-1β, and caspase-1 expression in rat hippocampus and cerebral cortex. A, G Western blots of NLRP3 expression in each group and (D, J) quantification of blot density. C, I Activation of casepase-1 and (B, H) maturation of pro-IL-1β in each group, and (E, K, F, L) quantification of blot density. β-Actin served as an internal control. Data are expressed as a percentage of control (mean ± SEM) for four mice/group. n = 4, **P < 0.01 vs control group; ##P < 0.01 vs 1-BP group.
Discussion
The MWM test is a major tool for measuring spatial memory in rats, and is widely used in behavioral neuroscience [34]. Previous studies have demonstrated that impaired performance in the MWM test is associated with distinct brain regions including the striatum, hippocampus, basal forebrain, cerebellum, and cerebral cortex [7]. Among these regions, the hippocampus and cerebral cortex play important roles in processing and retrieving memory [35, 36]. In addition, previous work has revealed that the hippocampus is one of the areas sensitive to 1-BP-induced toxicity [37]. Hence, here we focused on the hippocampus and cerebral cortex to investigate the protective effects of MK801 against 1-BP-induced neurotoxicity. Our results showed that exposure to 1-BP for 14 consecutive days significantly impaired the spatial learning and memory in rats, and this impairment was attenuated by MK801. Mechanistic investigation revealed that the interactions between NMDARs and microglial activation are essential for the neuroprotection afforded by MK801.
Information processing requires a delicate balance of excitatory and inhibitory signaling during memory encoding [38]. It is known that NMDARs, via excitatory signaling, play an important role in learning and memory formation [39, 40]. However, dysfunction of NMDARs is detrimental to learning and memory behaviors, and this has been implicated in the pathophysiology of brain disorders including AD [15, 18] and Parkinson’s disease (PD) [41]. The function of NMDARs is regulated by the balance of endogenous excitatory and inhibitory amino-acids. Glu and Asp are excitatory neurotransmitters that can bind to NMDARs, resulting in an increased intracellular Ca2+ concentration and thus, excitotoxicity, a common mechanism shared by several neurodegenerative disorders [42, 43]. GABA and Tau are the main inhibitory transmitters in the brain, and can modify neuronal responses to excitatory inputs by reducing cell excitability [43, 44]. Both GABA and Tau are capable of antagonizing Glu-mediated excitatory responses by reducing the binding of Glu to NMDAR subtypes [45]. Previous studies have shown that 1-BP exposure enhances neuronal excitability in the hippocampus through the over-activation of NMDARs [23, 46]. Consistent with previous reports, we found that the concentrations of GABA and Tau were significantly decreased in 1-BP-intoxicated rats [47]. Meanwhile, 1-BP intoxication increased the levels of excitatory Asp in rats, although the levels of Glu remained unchanged. Notably, the Glu content measured in the brain tissue of rats may not be able to exactly reflect the functional Glu in synaptic junctions. Unfortunately, detecting Glu in synaptic junctions using micro-dialysis was not available to us. In contrast to the unchanged Glu, we found that the concentrations of Gly were markedly elevated in 1-BP-intoxicated rats. Gly is a co-agonist required for the activation of NMDARs, apart from Glu and Asp. Gly is also known to decrease the desensitization of NMDARs [48]. Interestingly, in mice treated with both 1-BP and MK801, the changes in Gly and Asp induced by 1-BP recovered to a level similar to the control group.
We also assessed the expression of the NMDAR subunits GluN1, GluN2A, and GluN2B. Consistent with the increase in Gly, the expression of GluN1 was higher in 1-BP-treated rats. The GluN1 subunit is essential for channel formation and is the binding site for Gly, which is a co-agonist for NMDAR activation [49, 50]. Functional NMDA channels are heteromeric tetramers of GluN1 and GluN2A–D subunits. Different NMDAR isoforms formed by diverse combinations of GluN2 subunits serve unique roles in the brain. GluN2A/B is critical for synaptic plasticity and memory formation, and the phosphorylation of GluN2A/B enhances the ability of NMDARs to regulate Ca2+ influx [40]. Here, we found that 1-BP treatment resulted in decreased of GluN2A and GluN2B expression, but increased their phosphorylation levels in the brains of rats. These alterations in NMDAR subunits were abrogated by MK801 pretreatment. The results suggested that the effects of MK801 might be mediated partially by reducing NMDAR activation.
Mechanistically, the protection against 1-BP-induced neurotoxicity by MK801 might be related to its anti-inflammatory effect. Neuroinflammation mediated by microglia is gradually being recognized to be essential for neurodegeneration in response to exogenous insults [51]. In response to stimulation, microglia are readily activated and release pro-inflammatory cytokines, prostaglandins, and reactive oxygen/nitrogen species, which work together to cause neuronal damage [52]. Recent studies have demonstrated that microglial over-activation accounts for the cognitive dysfunction in patients with chronic neurodegeneration such as PD and AD [53, 54]. Accumulating evidence suggests cross-talk between NMDARs and microglial activation. NMDARs are expressed in microglial cells and have been implicated in their activation. Studies have shown that administration of NMDA to rats stimulates microglial activation and the secretion of pro-inflammatory factors, such as IL-1β and TNFα [19]. Blockade of NMDARs has been found to decrease microglial activation in a rat model of peripheral inflammatory pain [55]. In turn, activated microglia can stimulate the NMDARs expressed in both microglia and neurons through the release of a variety of pro-inflammatory factors [56], leading to further microglial activation and neuronal excitotoxicity. It has been demonstrated that MK801 protects against lipopolysaccharide-induced neuronal damage by inhibiting microglial activation [27]. Thus, we speculated that 1-BP exposure might trigger microglial activation and propagate a toxic interaction between NMDARs and activated microglia. The beneficial effects of MK801 against 1-BP-induced neurotoxicity might be attributed to the alleviation of neuroinflammation.
The pro-inflammatory cytokine IL-1β is critical for the activation of NMDARs and microglia [57]. IL-1β is a potent agonist of microglial activation that can stimulate the abundant production of nitric oxide and TNFα, as well as the activation of NMDARs [58]. Moreover, an in vitro study revealed that neurons exposed to IL-1β show increased postsynaptic entry of Ca2+ [57]. The NLRP3 inflammasome, a multiprotein complex, can sense various stimuli and form a molecular platform for caspase-1 activation, which leads to the production and release of IL-1β [59]. As illustrated in the present study, 1-BP intoxication resulted in increased NLRP3 expression and the cleavage of both pro-caspase-1 and pro-IL-1β, which was abolished by MK801. The results suggested that the NLRP3 inflammasome and related production of IL-1β might bridge the interaction between NMDARs and activated microglia.
In summary, the present study revealed that MK801 protects against 1-BP-induced cognitive dysfunction by ameliorating NMDAR action and blocking microglial activation. This expands the understanding of the pathogenesis of 1-BP-induced neurotoxicity and provides potential targets for the treatment of 1-BP poisoning. However, other mechanisms cannot be excluded in the protection afforded by MK801, since MK801 has versatile pharmacological properties. Further mechanistic studies are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81872654, 81703264); Fundamental Research Funds of Shandong University (2016JC020), China, and Natural Science Foundation of Shandong Province (ZR2017MH002), China.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Qingshan Wang, Email: wangq4@126.com.
Xiu-Lan Zhao, Email: zhao.xl@sdu.edu.cn.
References
- 1.Kim Y, Park J, Moon Y. Hematopoietic and reproductive toxicity of 2-bromopropane, a recently introduced substitute for chlorofluorocarbons. Toxicol Lett. 1999;108:309–313. doi: 10.1016/S0378-4274(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 2.Moon HI, Shin S, Byeon SH. Exposure monitoring and health risk assessment of 1-bromopropane as a cleaning solvent in the workplace. Hum Ecol Risk Assess. 2015;21:744–752. doi: 10.1080/10807039.2014.926203. [DOI] [Google Scholar]
- 3.Wang TH, Wu ML, Wu YH, Tsai WJ, Lin KP, Wang CL, et al. Neurotoxicity associated with exposure to 1-bromopropane in golf-club cleansing workers. Clin Toxicol. 2015;53:823–826. doi: 10.3109/15563650.2015.1064939. [DOI] [PubMed] [Google Scholar]
- 4.Samukawa M, Ichihara G, Oka N, Kusunoki S. A case of severe neurotoxicity associated with exposure to 1-bromopropane, an alternative to ozone-depleting or global-warming solvents. Arch Intern Med. 2012;172:1257–1260. doi: 10.1001/archinternmed.2012.3987. [DOI] [PubMed] [Google Scholar]
- 5.Ichihara G, Kitoh J, Yu X, Asaeda N, Iwai H, Kumazawa T, et al. 1-Bromopropane, an alternative to ozone layer depleting solvents, is dose-dependently neurotoxic to rats in long-term inhalation exposure. Toxicol Sci. 2000;55:116–123. doi: 10.1093/toxsci/55.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Sohn YK, Suh JS, Kim JW, Seo HH, Kim JY, Kim HY, et al. A histopathologic study of the nervous system after inhalation exposure of 1-bromopropane in rat. Toxicol Lett. 2002;131:195–201. doi: 10.1016/S0378-4274(02)00051-6. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Yuan H, Jiang LL, Yang JL, Zeng T, Xie KQ, et al. Involvement of decreased neuroglobin protein level in cognitive dysfunction induced by 1-bromopropane in rats. Brain Res. 2015;1600:1–16. doi: 10.1016/j.brainres.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Zhong ZX, Zeng T, Xie KQ, Zhang CL, Chen JJ, Bi Y, et al. Elevation of 4-hydroxynonenal and malondialdehyde modified protein levels in cerebral cortex with cognitive dysfunction in rats exposed to 1-bromopropane. Toxicology. 2013;306:16–23. doi: 10.1016/j.tox.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Chalupka S. Reducing workplace exposure to 1-bromopropane. Workplace Health Saf. 2014;62:128. doi: 10.1177/216507991406200308. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara G, Li W, Shibata E, Ding X, Wang H, Liang Y, et al. Neurologic abnormalities in workers of a 1-bromopropane factory. Environ Health Perspect. 2004;112:1319–1325. doi: 10.1289/ehp.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara G, Miller JK, Ziolkowska A, Itohara S, Takeuchi Y. Neurological disorders in three workers exposed to 1-bromopropane. J Occup Environ Med. 2002;44:1–7. doi: 10.1097/00043764-200201000-00001. [DOI] [Google Scholar]
- 12.Majersik JJ, Caravati EM, Steffens JD. Severe neurotoxicity associated with exposure to the solvent 1-bromopropane (n-propyl bromide) Clin Toxicol (Phila) 2007;45:270–276. doi: 10.1080/15563650701226218. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Ichihara G, Kitoh J, Xie Z, Shibata E, Kamijima M, et al. Neurotoxicity of 2-bromopropane and 1-bromopropane, alternative solvents for chlorofluorocarbons. Environ Res. 2001;85:48–52. doi: 10.1006/enrs.2000.4226. [DOI] [PubMed] [Google Scholar]
- 14.Soto D, Altafaj X, Sindreu C, Bayes A. Glutamate receptor mutations in psychiatric and neurodevelopmental disorders. Commun Integr Biol. 2014;7:e27887. doi: 10.4161/cib.27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi H, Sze CI. N-methyl-D-aspartate receptor subunit NR2A and NR2B messenger RNA levels are altered in the hippocampus and entorhinal cortex in Alzheimer’s disease. J Neurol Sci. 2002;200:11–18. doi: 10.1016/S0022-510X(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Ni W, Zhang C. Calcium-impermeable NMDA receptor: a novel target for addiction. Neurosci Bull. 2017;33:357–358. doi: 10.1007/s12264-017-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 18.Sze C, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci. 2001;182:151–159. doi: 10.1016/S0022-510X(00)00467-6. [DOI] [PubMed] [Google Scholar]
- 19.Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rammes G, Mattusch C, Wulff M, Seeser F, Kreuzer M, Zhu K, et al. Involvement of GluN2B subunit containing N-methyl-D-aspartate (NMDA) receptors in mediating the acute and chronic synaptotoxic effects of oligomeric amyloid-beta (Aβ) in murine models of Alzheimer’s disease (AD) Neuropharmacology. 2017;123:100–115. doi: 10.1016/j.neuropharm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang M, Li J, Tiu SC, Zhang L, Wang M, Yew DT. N-methyl-D-aspartate receptor and apoptosis in Alzheimer’s disease and multiinfarct dementia. J Neurosci Res. 2005;81:269–274. doi: 10.1002/jnr.20558. [DOI] [PubMed] [Google Scholar]
- 23.Fueta Y, Ishidao T, Arashidani K, Endo Y, Hori H. Hyperexcitability of the hippocampal CA1 and the dentate gyrus in rats subchronically exposed to a substitute for chlorofluorocarbons, 1-bromopropane vapor. J Occup Health. 2002;44:156–165. doi: 10.1539/joh.44.156. [DOI] [Google Scholar]
- 24.Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. doi: 10.3389/fncel.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang X, Sun D, Wang Z, Yu Z, Liu W, Pu Y, et al. MiR-30a positively regulates the inflammatory response of microglia in experimental autoimmune encephalomyelitis. Neurosci Bull. 2017;33:603–615. doi: 10.1007/s12264-017-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 28.Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian K, Mohideen SS, Suzumura A, Asai N, Murakumo Y, Takahashi M, et al. Exposure to 1-bromopropane induces microglial changes and oxidative stress in the rat cerebellum. Toxicology. 2012;302:18–24. doi: 10.1016/j.tox.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Han EH, Yang JH, Kim HK, Choi JH, Khanal T, Do MT, et al. 1-Bromopropane up-regulates cyclooxygenase-2 expression via NF-kappaB and C/EBP activation in murine macrophages. Food Chem Toxicol. 2012;50:1616–1622. doi: 10.1016/j.fct.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Xu YP, Wang S, Jiang LL, Wang H, Yang YL, Li M, et al. Identify melatonin as a novel therapeutic reagent in the treatment of 1-bromopropane(1-BP) intoxication. Medicine. 2016;95:8. doi: 10.1097/MD.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Irving G, Jiang L, Wang H, Li M, Wang X, et al. Oxidative stress mediated hippocampal neuron apoptosis participated in carbon disulfide-induced rats cognitive dysfunction. Neurochem Res. 2017;42:583–594. doi: 10.1007/s11064-016-2113-8. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Wang R, Jiang Q, Wang S, Yao Y, Shao L. Determination of amino acid neurotransmitters in rat hippocampi by HPLC-UV using NBD-F as a derivative. Biomed Chromatogr. 2014;28:459–462. doi: 10.1002/bmc.3062. [DOI] [PubMed] [Google Scholar]
- 34.Pini RT, do Vales LD, Braga Costa TM, Almeida SS. Effects of cafeteria diet and high fat diet intake on anxiety, learning and memory in adult male rats. Nutr Neurosci. 2017;20:396–408. doi: 10.1080/1028415X.2016.1149294. [DOI] [PubMed] [Google Scholar]
- 35.Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol. 1986;20:472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- 36.Sze CI, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. J Neurol Sci. 2000;175:81–90. doi: 10.1016/S0022-510X(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 37.Mohideen SS, Ichihara S, Banu S, Liu F, Kitoh J, Ichihara G. Changes in neurotransmitter receptor expression levels in rat brain after 4-week exposure to 1-bromopropane. Neurotoxicology. 2009;30:1078–1083. doi: 10.1016/j.neuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- 39.Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez A, Arbuckle MR. The N-methyl-D-aspartate receptor: memory, madness, and more. Biol Psychiatry. 2017;82:e1–e3. doi: 10.1016/j.biopsych.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallett PJ, Dunah AW, Ravenscroft P, Zhou S, Bezard E, Crossman AR, et al. Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Neuropharmacology. 2005;48:503–516. doi: 10.1016/j.neuropharm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Flavin HJ, Seyfried TN. Enhanced aspartate release related to epilepsy in (EL) mice. J Neurochem. 1994;63:592–595. doi: 10.1046/j.1471-4159.1994.63020592.x. [DOI] [PubMed] [Google Scholar]
- 43.Bradford HF. Glutamate, GABA and epilepsy. Prog Neurobiol. 1995;47:477–511. doi: 10.1016/0301-0082(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 44.Jia N, Sun Q, Su Q, Dang S, Chen G. Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1alpha pathway. Redox Biol. 2016;10:179–190. doi: 10.1016/j.redox.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan CY, Sun HS, Shah SM, Agovic MS, Ho I, Friedman E, et al. Direct interaction of taurine with the NMDA glutamate receptor subtype via multiple mechanisms. Adv Exp Med Biol. 2013;775:45–52. doi: 10.1007/978-1-4614-6130-2_4. [DOI] [PubMed] [Google Scholar]
- 46.Fueta Y, Fukunaga K, Ishidao T, Hori H. Hyperexcitability and changes in activities of Ca2+/calmodulin-dependent kinase II and mitogen-activated protein kinase in the hippocampus of rats exposed to 1-bromopropane. Life Sci. 2002;72:521–529. doi: 10.1016/S0024-3205(02)02247-6. [DOI] [PubMed] [Google Scholar]
- 47.Suda M, Honma T, Miyagawa M, Wang RS. Alteration of brain levels of neurotransmitters and amino acids in male F344 rats induced by three-week repeated inhalation exposure to 1-bromopropane. Ind Health. 2008;46:348–359. doi: 10.2486/indhealth.46.348. [DOI] [PubMed] [Google Scholar]
- 48.Mayer ML, Vyklicky L, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 50.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 51.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 52.Alimonti A, Ristori G, Giubilei F, Stazi MA, Pino A, Visconti A, et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology. 2007;28:450–456. doi: 10.1016/j.neuro.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Le W, Wu J, Tang Y. Protective microglia and their regulation in Parkinson’s disease. Front Mol Neurosci. 2016;9:89. doi: 10.3389/fnmol.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XF, Thompson M, Xu YH. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Investig. 2016;96:496–507. doi: 10.1038/labinvest.2015.161. [DOI] [PubMed] [Google Scholar]
- 55.Bayer TA, Multhaup G. Involvement of amyloid beta precursor protein (AbetaPP) modulated copper homeostasis in Alzheimer’s disease. J Alzheimers Dis. 2005;8:201–206. doi: 10.3233/JAD-2005-8212. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–668. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- 57.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han RZ, Hu JJ, Weng YC, Li DF, Huang Y. NMDA receptor antagonist MK-801 reduces neuronal damage and preserves learning and memory in a rat model of traumatic brain injury. Neurosci Bull. 2009;25:367–375. doi: 10.1007/s12264-009-0608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.