Table 1.
In vitro activity of compounds 1a–p.
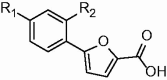 | |||||||
|---|---|---|---|---|---|---|---|
|
1a–i R1=CF3 |
1j–p R1=NO2 |
||||||
| Code | R2 | Residual activity at 100 µM (%) | MbtI IC50 (µM)* | Code | R2 | Residual activity at 100 µM (%) |
MbtI IC50 (µM)* |
| 1a | Cl | 6.1 ± 2.5 | 28.5 ± 2.6 | I** | Cl | 11.3 ± 4.2 | 17.9 ± 3.2 |
| 1b | F | 6.1 ± 1.1 | 27.6 ± 7.3 | 1j | F | 32.1 ± 2.4 | – |
| 1c | Br | 38.0 ± 3.7 | – | 1k | Br | 26.4 ± 4.8 | – |
| 1d | OH | 16.8 ± 2.7 | 35.9 ± 10.3 | 1l | OH | 13.9 ± 4.0 | 29.8 ± 4.2 |
| 1e | CH3 | 19.3 ± 2.2 | 31.5 ± 9.6 | 1m | CH3 | 13.2 ± 5.8 | 28.5 ± 1.5 |
| 1f | NH2 | 13.1 ± 2.4 | 34.6 ± 10.9 | 1n | NH2 | 13.5 ± 3.8 | 24.2 ± 5.4 |
| 1g | CN | 5.7 ± 1.5 | 18.5 ± 3.2 | 1o | CN | 5.0 ± 1.9 | 24.4 ± 5.9 |
| 1h | CF3 | 3.9 ± 1.7 | 13.1 ± 2.0 | 1p | CF3 | 25.1 ± 3.7 | 41.8 ± 5.3 |
| 1i | m-CF3 | 64.3 ± 4.5 | – | – | – | – | – |
*Only for compounds with residual activity ≤25%; **new fluorimetric assay value determined for the sake of comparison.
